Supplier Updates
Filters
 Supplier Name
Supplier Name
 Communication Type
Communication Type
 Date Range
Date Range
-
12/9/2025
Title: Abbott Nutrition V#10575
Details:
Dear Concordance Team,
We are sharing with all of our distributor partners the attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Communication Type:
Product Inventory Update
V#10575
-
12/9/2025
Title: CHS USA Incorporated V#10379
Details:
Hello,
Please see the upcoming holiday calendar for CHS USA V#10379. Thanks!
Please note there have been some updates to the closure dates at CHS USA:
*** OPEN – Wednesday December 24th ***
** OPEN – Wednesday December 31st ***CHS USA Holiday Closure Notice:
CHS USA will be closed on the following dates for the holidays:
Thursday December 25 – Christmas
Friday December 26
Thursday January 1 – New YearsPlease keep in mind these dates when planning your purchase order requirements for the following months and please be sure to let your CHS USA Sales Representative know should you have any questions or concerns.
Additionally, you can also reach out to our CHS USA Customer Care Department at 1-888-986-0042.
Sincerely,
CHS USA Incorporated
200 Kelly Drive, Suite A
Peachtree City, GA
30269Communication Type:
Holiday Schedule
V#10379
-
12/9/2025
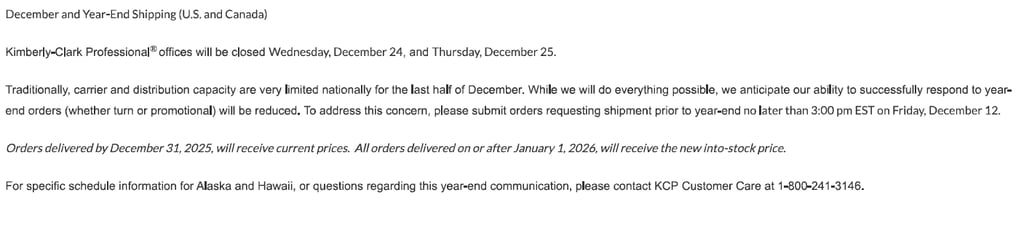
Title: Kimberly Clark V#10765
Details:
Hi, everyone.
Please see attached from Kimberly Clark regarding their end of year shipping schedule.
Communication Type:
Holiday Schedule
V#10765
-
12/9/2025
Title: Central Solutions V#11992
Details:
Hello,
Please see the notice below from Central Solutions V#11992. Please contact Mary Reinhart if you have questions. A usage file for the impacted items is attached.
Dear Valued Customers,
We hope you are enjoying a wonderful holiday season.
We are writing to share that over the next several months, we will be rebranding our line of bathing and skin care liquids. Part of this effort requires strategically depleting inventory of the SKUs listed below, and offering DermaCen ApraCare and DermaCen Hand Sanitizer as substitutes.
If you have any open orders for DermaCen Freesia Shampoo 9oz (23062) or DermaCen Melon Breeze Shampoo (23162), please let us know whether you would prefer to substitute with the recommended alternative or cancel your existing order.
For your convenience, I have provided below a list of those SKUs impacted by this rebrand, and our recommended replacements.Deactivating Once Stock is Depleted
Replacement Item
SKU #
Description
Size
Current Inventory 12/4/2025
SKU #
Description
Size
23061
DERMACEN FREESIA SHAMPOO & BODY WASH
1 GALLON
15 Cases
23051
DERMACEN APRA CARE SHAMPOO & BODY WASH
1 GALLON
23062
DERMACEN FREESIA SHAMPOO & BODY WASH
9 OZ
0 Cases
23052
DERMACEN APRA CARE SHAMPOO & BODY WASH
9 OZ
23162
DERMACEN MELON BREEZE SHAMPOO & BODY WASH
9 OZ
0 Cases
23052
DERMACEN APRA CARE SHAMPOO & BODY WASH
9 OZ
23161
DERMACEN MELON BREEZE SHAMPOO & BODY WASH
1 GALLON
11 Cases
23051
DERMACEN APRA CARE SHAMPOO & BODY WASH
1 GALLON
23166-1250
DERMACEN MELON BREEZE SHAMPOO & BODY WASH
1250 ML
36 Cases
23056-1250
DERMACEN APRA CARE SHAMPOO & BODY WASH
1250 ML
22964
DERMACEN TEARLESS SHAMPOO & BODY WASH
12 OZ
0 Cases
23052
DERMACEN APRA CARE SHAMPOO & BODY WASH
9 OZ
14013
DERMACEN INSTANT HAND SANITIZER W/ ALOE
8 OZ
73 Cases
14017
DERMACEN INSTANT HAND SANITIZER W/ALOE
18 OZ
Please note that more information on our exciting rebranding efforts will be available in the near future.
As always please feel free to reach out with any questions.
Communication Type:
Discontinuation Notice
V#11992
Communication Type:
Product Changes
V#11992
-
12/9/2025
Title: Molnlycke V#10008
Details:
Mepi Press 2 Launch Distributor Notification
Condordance Team,
MESSAGE FROM MOLNLYCKE V#10008:
Dear Valued Distributor Partner:
Mölnlycke® is proud to announce the launch of our new Mepi™ Press 2 and Mepi™ Press 2 Lite. These multi-component compression wraps will be available in January 2026.
Ordering details:
Please see below for product details:
Product Code
Product Description
List Price / CASE
300031
Mepi Press 2 Lite – 8 sets per box
$236.00
300021
Mepi Press 2 – 8 sets per box
$180.00
Attached for your review is our official Launch Notification, which provides product specifications and pricing as illustrated above, and our product brochures for these 2 new products. Please contact your local representative or call the Mölnlycke Customer Care at 1-800-843-8497 for any additional information you may require.
Thank you for your support; we look forward to continuing to help meet your wound care needs.
Communication Type:
New Product Launch
V#10008
-
12/8/2025
Title: Nestle V#10183
Details:
Hello,
Please see below from Nestle V#10183.
Attached is the Inventory letter for this week.
1. Benecalorie still on the report
2. BOOST GLUCOSE CONTROL® MAX 30 g PROTEIN, Chocolate, 3 (4 x 11 fl oz) carton – Retail still on the report
3. BOOST GLUCOSE CONTROL® MAX 30 g PROTEIN, Vanilla, 3 (4 x 11 fl oz) carton – Retail still on the report
4. COMPLEAT® PEPTIDE 1.5 Cal, SpikeRight® PLUS 6 x 1000 mL UltraPak® bag still on the report
5. COMPLEAT® PEDIATRIC STANDARD 1.4 Cal, Vanilla 24 x 250 mL carton still on the report
6. BOOST®, Very Vanilla 24 x 8 fl oz bottle were added to the report
7. NUTRISOURCE® FIBER, Unflavored Powder 4 x 7.2 oz canisters were added to the report
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Communication Type:
Product Inventory Update
V#10183
-
12/8/2025
Title: Steris V#10863
Details:
Hello,
Please see the discontinuation notice from Steris V#10863. There has been no usage on the items being discontinued.
Dear Valued Distributor,
This communication is to inform you that effective December 15, 2025, STERIS will no longer accept orders for the following products (subject to inventory availability):
Discontinued
ItemDiscontinued Description
Replacement
ItemItem Description
UOM
Quantity Per UOM
Acquisition Cost (2025 and 2026)
Status
4505
FACE SHIELDS FULL 13 INCH WIDE 7.5 INCH LONG
[24/BX]4505B
FACE SHIELDS FULL 13 INCH WIDE 7.5 INCH LONG
[24/BX]BX
24
62.10
Inventory available
4506
FACE SHIELDS FULL 13 INCH WIDE 9 INCH LONG
[24/BX]4506B
FACE SHIELDS FULL 13 INCH WIDE 9 INCH LONG
[24/BX]BX
24
64.40
No longer available
4509
FACE MASK VISOR WITH SPLASH GUARD [24/PK]
4509B
FACE MASK VISOR WITH SPLASH GUARD [24/BX]
BX
24
94.30
Inventory available until 12/15/25 (estimate)
At this time, inventory of 4506 has been depleted. Any open purchase order lines for 4506 will be cancelled.
If Customers have questions regarding this change, they should contact their local STERIS sales representative.
Thank you for your continued partnership.
Communication Type:
Discontinuation Notice
V#10863
-
12/8/2025
Title: J&J V#10196
Details:
Hello,Please see the update below and attached from J&J V#10196. Per their email:Effective February 1, 2026, the product code for US Mersilene tape, RS21, willchange to our global product code, RS21XN. This change only affects packaginglabels; the product, specifications, and performance remain the same.Ordering Actions Required:• Add RS21XN: Update your ordering system accordingly to reflect the newproduct code RS21XN and begin ordering this code effective February 1,2026.• RS21 Transition: Product code RS21 will no longer be available forordering as of February 1, 2026.Communication Type:
Product Changes
V#10196
Communication Type:
Label Changes
V#10196
-
12/5/2025
Title: Solventum V#10296
Details:
Hello,Please see Solventum’s message below about mfg #9216 (C#177643) being on allocation, the timeline, and the impact on open orders.
Update on 9216 Avagard Surgical Hand Antiseptic, 1200 mL, 4 bottles/case
- We have stock this week for Avagard Surgical 9216 1200 mL, and will be releasing open PO’s later this week or early next week.
- The allocation to be released is approximately a 4-month supply based on historical usage. We expect to have the next batch of product available around March.
- Based on this, we suggest to continue to manage 9216 on allocation for ongoing customers for this product. Please note that in general, there is not enough supply of 9216 to be able to be a sub for Avagard 9200.
- The list of Concordance PO’s being released is attached. Note: This uses all of Concordance’s allocation for December-February. Open PO’s would be pushed out until around March when we expect to be back in stock for this item.
Please contact Tricia Wentling if you have any questions. Thanks!Communication Type:
Allocation Notification/Update
V#10296
-
12/5/2025
Title: New World Imports V#11823
Details:
Hello,Please see below and attached from New World Imports V#11823. C#291387 is being discontinued. I have attached a usage file for the last six months. Please reach out to Heather Burkett if you have any questions. Thanks!Communication Type:
Discontinuation Notice
V#11823
-
12/4/2025
Title: Roche V#10603
Details:
Concordance Team,
From Roche Diagnostics V#10603:
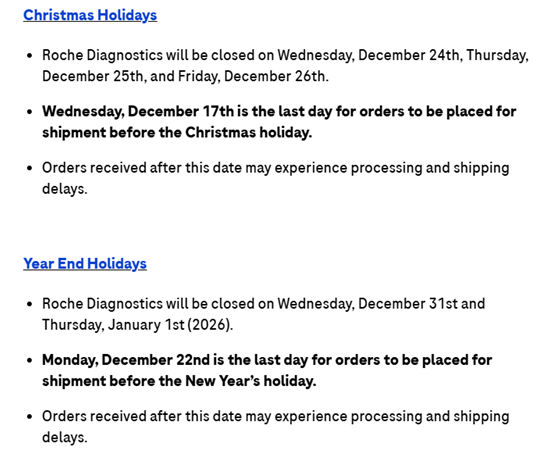
Thanks! Please let me know if you have any questions!
Shelley Miller
National Account Manager, Point of Care
Roche Diagnostics Corporation
9115 Hague Road
Indianapolis, IN 46250-0457
Communication Type:
Holiday Schedule
V#10603
-
12/4/2025
Title: Medegen V#10423
Details:
Hello,
Please see the upcoming holiday schedule below for Medegen V#10423. Please reach out to Tricia Wentling if you have any questions.
Please be advised that our facilities will be closed for shipping on the following dates during the 2025 holiday season. We encourage you to submit your orders as soon as possible to ensure timely delivery.
NO SHIP DATES
Gallaway, TN Facility
Clarksburg, WV Facility
12/24/25 - 12/26/25
12/24/25 – 12/26/25
12/31/25 - 1/2/26
1/1/26
For any inquiries, please do not hesitate to contact our customer service department at 1-800-511-6298 or cservice@medegenmed.com.
As always, thank you for your business and continued support! Happy Holidays!
Communication Type:
Holiday Schedule
V#10423
-
12/3/2025
Title: NDC V#10030
Details:
Hello,
Please see the holiday schedule for NDC V#10030 below:
NDC Holiday Shipping Schedule
Our offices and distribution centers will be closed on December 25, 2025, and January 1, 2026.
With the continued strain on the transportation carriers, we are encouraging all customers to order early to ensure timely deliveries.
We will accept orders on December 24, December 26 and December 31 until noon CT. Customer Service will be closing at 2:00 pm CT on December 24, December 26 and December 31.
If your normal order day is Thursday, for your December 25 and January 1 order, please place your order by Wednesday at noon CT on December 24 and December 31.
Please note that some shipments scheduled to leave on December 24, December 26 and December 31 may be delayed.
The LTL carriers are closed December 25, 2025, and January 1, 2026. We expect significant delays and encourage customers to order the week of December 15 if possible.
Other Important Notes- The projected arrival days are estimates that reflect the expected holiday delays with freight carriers. We cannot guarantee arrival dates or times, particularly with the holidays.
- Please order any product that is subject to freezing by December 19 to avoid additional cost or potential issues. There are freezing conditions projected in the upcoming weeks.
- FedEx and UPS Parcel shipments will not move on December 25, 2025, or January 1, 2026. We also anticipate heavy loads in parcel that will result in delays. Please plan accordingly.
- If you have questions or want to check on estimated delivery dates of your shipment, there are three different ways to do so:
- Access the NDC MemberSite > Products > Shipment Tracking
- Email memberinquiries@ndc-inc.com
- Call Customer Service at 877.436.5378
Any emails or orders submitted on December 25, 2025, and January 1, 2026, will be processed on the following business day. Please contact Customer Service at 866-632-2282 with any questions and send any closure notices to faciltyclosurenotice@ndc-inc.com.
We extend to all our partners a safe and joyous holiday season!Communication Type:
Holiday Schedule
V#10030
-
12/3/2025
Title: Nestle V#10183
Details:
Hello,
Please see the update below and attached from Nestle V#10183. The updates are to the COMPLEAT® Organic Blends and COMPLEAT® Pediatric Organic Blends and will take place in February 2026.
Nestle Health Science Distributor Communication Letter 12.01.2025
Compleat Pediatric Organic Blends Side-by-Side November 2025
Compleat Organic Blends Side-by-Side November 2025
Compleat Organic Blends and Boost Original Letter Spec Sheet as of 11.21.2025 Letter Version
Communication Type:
New Product Launch
V#10183
Communication Type:
Product Changes
V#10183
-
12/3/2025
Title: Minigrip V#13218
Details:
Concordance Team,
A message from Minigrip V#13218:
Hello,
Please be advised that our Houston, Texas facility will be closed for shipping on the following dates during the 2025 holiday season:
Wednesday, 12/24/25
Thursday, 12/25/25
Thursday, 1/1/2026
To ensure timely delivery, we encourage you to submit your orders as soon as possible. For any inquiries, please do not hesitate to contact our customer service department, 1-800-533-1931 or mgservice@medegenmed.com.
As always thank you for your business and continued support.
Happy Holidays!
Customer Service Department
Inteplast Group – Minigrip
360 Motor Parkway, Suite 800
Hauppauge, NY 11788Communication Type:
Holiday Schedule
V#13218
-
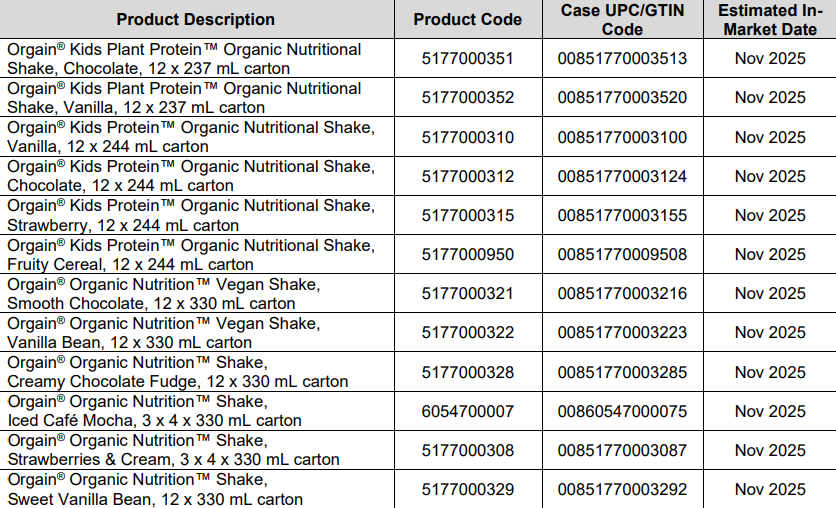
12/2/2025
Title: Nestle V#10183
Details:
Hello,
Please see the new product flyers from Nestle V#10183.
NEST-15612-1025_132725 Orgain Adult Detailed Aid-CROPS-BLEEDS-PDFX4 (1)
Orgain_Adult_Kids_Detail_Aid_Products_080625
NEST-15606-0925_OrgainKids_DetailAid
Communication Type:
Product Flyer
V#10183
Communication Type:
New Product Launch
V#10183
-
12/2/2025
Title: Lifesign V#12829
Details:
Hello,
Please see below and attached from Lifesign V#12829.
LifeSign/PBM now has been 510K Cleared for our Status Covid-19/FLU panel test. This also comes with an industry leading 28 month shelf life dating.
Communication Type:
Product Update
V#12829
-
12/1/2025
Title: BD V#10125
Details:
Hello,Please see below and attached from BD V#10125. They have asked that we replace mfg#305766 (C#345686) with mfg#305712 (362252). I have attached a usage report for mfg#305766 (C#345686) that we should no longer be ordering. Please contact Heather Burkett if you have questions. Thanks!Communication Type:
Substitution Option
V#10125
-
12/1/2025
Title: Owens & Minor V#12264
Details:
Hello,
Please see the holiday schedule below from Owens and Minor V#12264.
Valued Customer,
We would like to inform you that Owens & Minor Distribution Centers will be closed on Thursday, January 1 in observance of the New Year's Day holiday.
Please note:If you normally receive your orders on Thursdays unless previous arrangements have been made, all deliveries will be made on your next scheduled delivery day following the holiday. We ask that you adjust your orders accordingly.
Reach out to your Customer Service Team by end of day on Friday, December
19, 2025 to request alternative arrangements. The Customer Service Team will work with the shipping DC to determine if the request can be accommodated. Additional fees may be applied.
If no special delivery is requested and arranged, all freight and fees for off-day deliveries will apply.
Thank you for your partnership and have a safe holiday!Communication Type:
Holiday Schedule
V#12264
-
11/26/2025
Title: Solventum V#10296
Details:
Hello,Please see the update from Solventum V#10296. Solventum is introducing updated designs of the Bair Hugger™ underbody warming blankets to increaseproduction capacity and improve service levels. Production of the new blankets began in November 2025, withchanges - including a new catalog code - expected to take effect in mid-December 2025. I have attached a usage file for the last six months.Per Kris' instructions, if you could load the new codes BUT WAIT to go live until we give you notice, we would appreciate it.
Please reach out to Tricia Wentling if you have any questions. Thanks!
DISTRIBUTION Underbody Blanket Bair Hugger Details_November 2025
Communication Type:
Discontinuation Notice
V#10296
-
11/26/2025
Title: Coloplast V#10581
Details:
Hello,Please see the update below and attached from Coloplast V#10581. I have attached a usage file for the items included on the list.The addition of the new HCPCS codes for Hydrophilic Intermittent Catheters is right around the corner. To promote awareness and support users Coloplast is executing direct to consumer campaigns which include:
- Social Media
- Direct mail campaign
- Email campaign
- Text campaign
In addition, Coloplast will be putting stickers on our boxes of catheters as well as an insert in the box to support end users of our products. Attached is a copy of the sticker, the insert as well as an FAQ document which may be helpful for your teams who may receive questions.
Hydrophilic_Intermittent_Urinary_Catheter_Crosswalk_PM-40088_F
Communication Type:
Label Changes
V#10581
-
11/26/2025
Title: Attends V#10055
Details:
Hello,Please see the update below from Attends V#10055. The two items below are on backorder until December 8th. I have attached a usage file for the last six months. Thanks!APPNT20
52630
ATTENDS EXTENDED WEAR OVERNIGHT PULL ON UNDERWEAR APPNT20 MEDIUM 4/16
12/8/2025
Out of stock
APPNT30
52631
ATTENDS EXTENDED WEAR OVERNIGHT PULL ON UNDERWEAR APPNT30 LARGE 4/14
12/8/2025
Out of stock
These are getting the New Updated Large core in the Back for more coverage.
Communication Type:
Backorder Notification/Update/Reports
V#10055
-
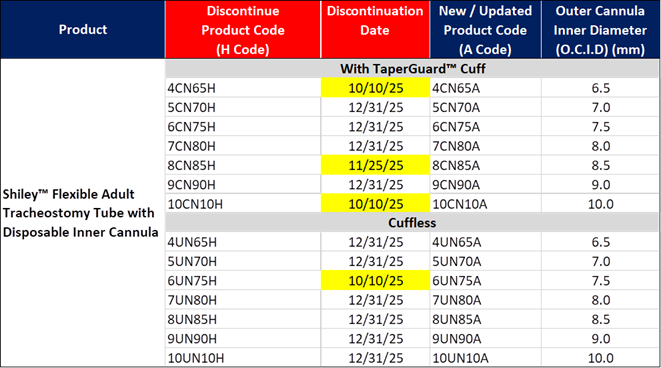
11/26/2025
Title: Medtronic V#10255
Details:
Hello,
Please see the update from Medtronic V#10255 below. Update: Product 8CN85H was discontinued on 11/25/2025. I have attached a usage file for the discontinued item.
Below is an update regarding the product code change to the Shiley™ flexible adult tracheostomy tubes with disposable inner cannulas.
- The product codes indicating a disposable inner cannula will transition from ending in H to ending in A.
- Please see the chart below with the updated discontinuation date for the H product codes.
Communication Type:
Discontinuation Notice
V#10255
-
11/26/2025
Title: Mead Johnson V#10502
Details:
Hello,
Please see the below and attached from Mead Johnson V#10502 regarding a discontinuation. A usage file is attached.
Attached is information on the discontinuation of Nutramigen 2oz. and 6oz. RTF and the replacement product Nutramigen 8oz RTF. This replacement will primarily affect hospitals. The 6oz. is estimated to deplete this month/next (which I assume the volume is very small or non-existence). The 2oz. is more widely used in hospitals and that product is estimated to deplete early Q1 2026.
It’s the same product but the big difference is the 8oz. has a much longer shelf life (12mos) vs. the others (6mos or less). Hospitals have been informed of the transition.
Communication Type:
Discontinuation Notice
V#10502
-
11/25/2025
Title: Ansell V#11003
Details:
Hello,
Please see the holiday schedule below and attached for Ansell V#11003.

Communication Type:
Holiday Schedule
V#11003
-
11/25/2025
Title: S2S Global V#10006
Details:
Hello,
Please see the upcoming holiday schedule below and attached for S2S Global V#10006.
Please be advised that S2S Global’s office and warehouses will be closed in observance of the following holidays.
We appreciate your understanding and are grateful for your ongoing partnership. We wish you a wonderful
holiday season!Thanksgiving (Closed November 27 and November 28, 2025)
• Last full shipping day is Tuesday, November 25th (Limited service on Wednesday, November 26th).
• S2S Global will resume normal operations on Monday, December 1st.Christmas (Closed December 25, 2025)
• Last shipping day is Tuesday, December 23rd with Limited Service.
• Please submit orders by Friday, December 12th in-order to ship by Tuesday, December 23rd.
• S2S Global will resume operation with limited services on Friday, December 26th.
• Normal operations will resume on Monday, December 29th.New Year’s Eve/Day (Closed January 1, 2026)
• Last shipping day is Tuesday, December 30th with Limited Service
• S2S Global will resume operation with limited services on Friday, January 2nd.
• S2S Global will resume normal operations on Monday, January 5th.If you have any questions, please contact our Customer Support Team at 1-855-531-7699 or via email at
osd_logistics@s2s-global.comCommunication Type:
Holiday Schedule
V#10006
-
11/24/2025
Title: Nestle V#10183
Details:
Hello,
Attached is the Weekly Inventory Letter for Nestle V#10183.
1. Benecalorie is still on the report
2. Boost Vanilla was added to the report
3. BOOST GLUCOSE CONTROL® MAX 30 g PROTEIN, Vanilla, 3 (4 x 11 fl oz) carton – added
4. BOOST GLUCOSE CONTROL® MAX 30 g PROTEIN, Chocolate, 3 (4 x 11 fl oz) carton - added
6. Compleat Peptide 1.5 1000 ml was added to the report
7. Thickenup Clear 4.4 oz canister is still on the report
8. THICKENUP® CLEAR, 12 (24 x 1.4g) packets
Communication Type:
Product Inventory Update
V#10183
-
11/24/2025
Title: Abbott Nutrition V#10575
Details:
Dear Concordance Team,
We are sharing with all of our distributor partners the attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Communication Type:
Product Inventory Update
V#10575
-
11/24/2025
Title: Health-o-Meter V#10075
Details:
Hello,
Please see below product release from Health-O-Meter V#10075 for your use and reference.
Please reach out to 'Brett Lucido' blucido@homscales.com for any questions.
Introducing the New BCS-G7 Body Composition Scale from Health o meter® Professional
Pelstar LLC is proud to unveil the BCS-G7 Body Composition Scale, the next evolution of our trusted BCS-G6 analyzer—now enhanced with integrated PC software for smarter, more efficient patient management.
The BCS-G7 provides a comprehensive body composition analysis with detailed segmental data for the torso and limbs. It distinguishes muscle mass vs. fat, empowering clinicians to ensure patients are truly losing fat and gaining muscle—not just watching the number on the scale.
This next-generation device is especially valuable for physicians monitoring patients on GLP-1 weight-loss medications, offering clear insights into treatment response and overall progress.
Paired with the new BodyCompPro–G7 App, users benefit from:
- Seamless data tracking
- Detailed reporting
- Streamlined patient management
- A simple, intuitive interface designed for busy clinical environments
With its combination of advanced analytics and easy workflow integration, the BCS-G7 is the ideal solution for weight-loss clinics, bariatric practices, medspas, and doctor’s offices seeking to elevate their patient care.
Learn More! https://www.homscales.com/bcs-g7/ or reach out to Brett Lucido, Director of National Accounts, blucido@homscales.com, (724) 799-4233 if you have any questions or would like more information.
Communication Type:
New Product Launch
V#10075
-
11/24/2025
Title: Airlife V#11179
Details:
Hello,Please see the discontinuation notice from Airlife V#11179. They have discontinued mfg# S5000N (C#122078) and have suggested mgf#9-0203-06 (C#428939) as a sub. Please reach out to Tricia Wentling if you have any questions. Thanks!
Communication Type:
Discontinuation Notice
V#11179
-
11/24/2025
Title: Medline V#10605
Details:
Hello,Please see the information from Medline V#10605 below. I have attached a usage report for the items that will be discontinued.We will be transitioning our current pre-inflated anesthesia masks to a new model designed to maintain the same high standards of patient care.
Key Points:
- The conversion process will be managed internally to ensure a seamless transition.
- Inventory and ordering adjustments will be handled by Medline’s operations team.
- Sales representatives can continue their current activities without disruption, as the new masks will automatically replace the existing versions.
Below are the new SKUs and estimated transition timelines based on current demand:

Communication Type:
Discontinuation Notice
V#10605
-
11/24/2025
Title: Essendant V#10572
Details:
Hello,Please see the upcoming holiday schedule for Essendant V#10572.Dear Customers,
In observance of the Thanksgiving holiday, Essendant will be closed on Thursday, November 27. This includes all departments and locations, including our customer care, distribution centers (DCs) and delivery services. Please note the following modified closing hours surrounding the holiday:
-
Wednesday, November 26
- All Essendant departments will be OPEN.
-
Thursday, November 27
- All Essendant departments, DCs, and Customer Care will be CLOSED.
- Carriers are NOT operating, and deliveries will resume on your NEXT SCHEDULED delivery day.
-
Friday, November 28
- All Essendant departments will be OPEN.
- Customer Care will be available for email support from 7AM – 5PM CST.
- Carriers operating and servicing Friday's scheduled deliveries.
- Staples will NOT be filling or shipping orders from their DCs.
Please note that our Customer Technical Support Team is available during closures. They can be reached during regular business hours at 1-800-733-5555.
We’re thankful for your business and will continue our efforts to deliver real, measurable results in terms of speed, reliability and value. Thank you for your continued support, and on behalf of the entire team at Essendant, we wish you and your families a safe and wonderful holiday. Happy Thanksgiving!
Essendant Leadership Team
Communication Type:
Holiday Schedule
V#10572
-
-
11/24/2025
Title: Medi USA V#10848
Details:
Hello,Please see the upcoming holiday schedule from Medi USA V#10848.Valued customer,
The busy holiday season is coming fast! Let’s work together to make sure your patients receive their custom medi® products before the end of the year. Below are the key order deadlines for shipments by December 31, 2025. Be advised - the first deadline is in less than two weeks!
For on-time delivery, please ensure all custom orders are fully completed and approved for production by:
2025 Custom Order Deadlines:
• Custom flat-knit trend colors – Tuesday, December 2
• Custom flat-knit standard colors – Tuesday, December 9
• Custom circular-knit – Wednesday, December 17
• Custom circaid® – Friday, December 26We value your partnership and are committed to supporting you and your patients as we head into the final stretch of 2025.
Communication Type:
Holiday Schedule
V#10848
-
11/20/2025
Title: Welch Allyn V#10834
Details:
CVSM 6400 and 6500 End of Service Letter
Concordance Team,
See the attached announcement from Welch Allyn V#10834 about CVSMs that were discontinued back in 2018.
This letter is to inform customers that Baxter (formerly Hillrom) will discontinue all technical phone support, service, repair, calibration, and parts support activities associated with our Welch Allyn Connex Vital Signs Monitor (CVSM) 6400 and 6500 Series effective December 31, 2025. As a reminder, they ended manufacturing of the CVSM 6400 and 6500 Series on September 30, 2018. They will continue to manufacture and service the CVSM 6700 and 6800 Series. A selection of the impacted part numbers is attached in Appendix A of this letter.
Communication Type:
Discontinuation Notice
V#10834
-
11/20/2025
Title: Attends V#10055
Details:
Hello,
Attends V#10055 sent us a few items that will be out of stock until December.
UFPP300 (C# 199784) until Dec 20.
Reason : we are waiting on a new screen part for the machine.
It will improve the quality & appearance of the underpads.
APPNT20 (C# 385115) until Dec. 11
We are improving the core to have a new wider back on it.
Giving folks more coverage for Overnight Protection.
I have attached a usage report for the last six months. Per Valeriya's email below, please see recommended HCS subs:We do not carry the underwear yet, however, please recommend to the Sales and CS the HCS underpad # 81709/CHS# 387587. Inventory is healthy and stocked in RIPL, TIFF, 1SIO, 2STL, 2SEC, 2PHX, and 2WIC.
Communication Type:
Temp Unavailable
V#10055
Communication Type:
Substitution Option
V#10055
-
11/20/2025
Title: BD V#10125
Details:
Hello,Per the emails below and attached, the items below have been removed from allocation by BD V#10125.CHS#312686
CHS#166243
Please reach out to Heather Burkett if you have questions. Thanks!Communication Type:
Allocation Notification/Update
V#10125
-
11/19/2025
Title: Kenvue Brands V#10860
Details:
Concordance Team,
Please see the Kenvue V#10860 holiday schedule below:
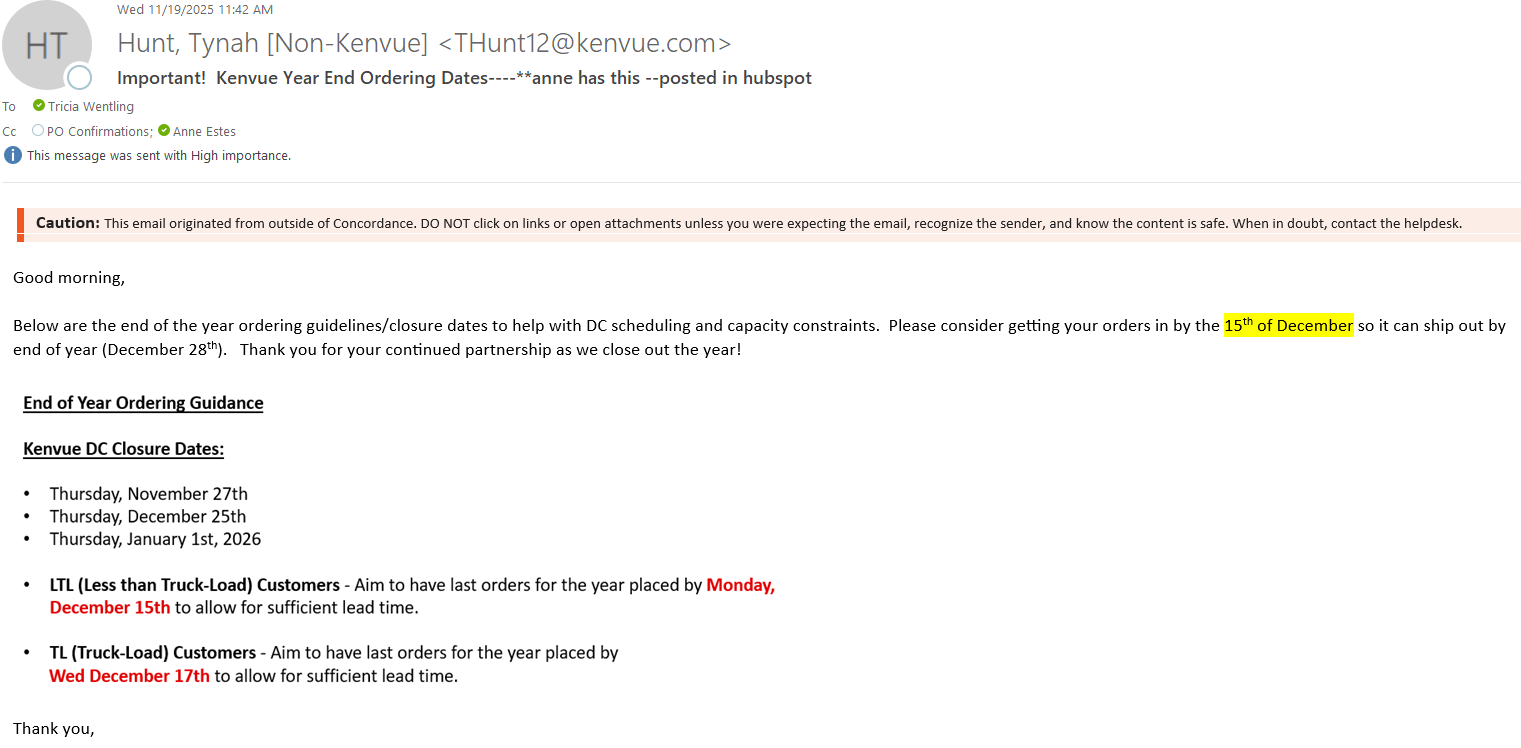
Communication Type:
Holiday Schedule
V#10860
-
11/18/2025
Title: BSN Medical V#10231
Details:
Hello,Please see the update below and attached from BSN Medical V#10231. Several products that have been on backorder will be available soon. Please see the full list of products and usage report attached.Product Date Available
Gypsona HP Week of November 17th
Gypsona S ExFast (XFST) Week of November 17th
Gypsona S Fast (FST) Week of December 8th
OCL Week of December 15th
Gypsona Elastic Week of December 15th
Communication Type:
Backorder Notification/Update/Reports
V#10231
-
11/18/2025
Title: Sage V#10229
Details:
Concordance Team,
Sage Products has announced a new product in the attachment.
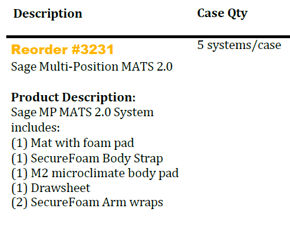
Communication Type:
Product Update
V#10229
-
11/17/2025
Title: Medtronic V#10255
Details:
Hello,Please see the discontinuation notice below and attached from Medtronic V#10255. I have attached a usage file for the last six months.As part of our commitment to continually improve our products, we have made the decision to discontinue the 1.0 Tri-Staple™ reloads and offer the 2.0 Tri-Staple™ reloads as your replacement SKU’s effective on February 1, 2026.
Attached:
Product To Be DiscontinuedC#Product DescriptionRevised Discontinue DateEGIA45AMT172501Tri-Staple™ 1.0 Reload 45mm Medium/Thick Tissue Purple2/1/2026EGIA60AMT172503Tri-Staple™ 1.0 Reload 60mm Medium/Thick Tissue Purple2/1/2026EGIA45AVM172499Tri-Staple™ 1.0 Reload 45mm Vascular Medium Tissue Tan2/1/2026EGIA60AVM172500Tri-Staple™ 1.0 Reload 60mm Vascular Medium Tissue Tan2/1/2026EGIA45AV195773Tri-Staple™ 1.0 Reload 45mm Extra Thin/Vascular Tissue Gray2/1/2026Communication Type:
Discontinuation Notice
V#10255
-
11/17/2025
Title: Cardinal V#10801
Details:
Hello,Please see the attached product inventory updates from Cardinal V#10801. There are several items with updated estimated recovery dates. Please reach out if you have questions. Thanks!Communication Type:
Allocation Notification/Update
V#10801
-
11/14/2025
Title: Airlife V#11179
Details:
Hello,Please see the discontinuation notice from Airlife V#11179. C#607638 (mfg #2K8030) has been discontinued. They did list a sub item #HS1030, HYPER SYS, 3L. I have attached a usage file for the last six months. Please reach out to Tricia Wentling if you have further questions. Thanks!
Communication Type:
Discontinuation Notice
V#11179
-
11/14/2025
Title: Pfizer V#12114
Details:
Hello,Please see the holiday schedule from Pfizer V#12114 below.Pfizer will be closed on Thursday, November 27, 2025 and Friday, November 28, 2025 in observance of the Thanksgiving holiday. Our Hemophilia operations will be impacted as follows:
Pfizer Holiday Schedule
Wednesday, November 26, 2025
Closing at 6:00pm EST
Thursday, November 27, 2025
Closed
Friday, November 28, 2025
Closed
Pfizer Holiday Shipping Schedule
Order Placed By
Expected Delivery By
Tuesday, November 25, 2025 before 4:30pm EST
Wednesday, November 26, 2025
Tuesday, November 25, 2025 after 4:30pm EST
Tuesday, December 2, 2025
Wednesday, November 26, 2025
Tuesday, December 2, 2025
We will return to our standard business schedule on Monday, December 1, 2025. As always, Pfizer will make every effort to accommodate emergency needs as they arise.
Communication Type:
Holiday Schedule
V#12114
-
11/14/2025
Title: Essity V#10272
Details:
Hello,Please see the notification attached from Essity V#10272. Per their email, Essity Tena products are now on the Premier Protective Underwear Contract # PP-NS-2129. Please reach out to Heather Burkett if you have questions. Thanks!
Communication Type:
Product Update
V#10272
-
11/14/2025
Title: Bemis V#10582
Details:
Hello,Please see the holiday schedule from Bemis V#10582 attached. Thanks!Please be advised that Bemis Manufacturing will be closed on the following
dates in observance of the holidays:
- November 27 & 28 - Thanksgiving Holiday
- December 24 & 25 - Christmas Holiday
- December 31 & January 1 -New Year's HolidayDuring these dates, our offices, production facilities, and shipping department
will be shut down. We encourage you to plan your orders and
communications accordingly to avoid any disruptions.
Thank you for your continued partnership. We wish you a joyful and safe
holiday season!Communication Type:
Holiday Schedule
V#10582
-
11/14/2025
Title: Cardinal V#10801
Details:
Hello,Please see the upcoming holiday schedule for Cardinal V#10801 below and attached. Thanks!Due to the upcoming holidays 12/25/2025, 1/1/2026, & 1/19/2026, your Cardinal Health replenishment centers will be closed for business. Please note the order cut offs below for the appropriate weeks.
For standing delivery appointments (SDAs), adjustments will be coordinated by your transportation replenishment center leader.- December 25
- Adjust any ordering that typically falls on Monday, 12/22/25, Tuesday, 12/23/25, Wednesday, 12/24/25 & Thursday, 12/25/25 to be ordered by Monday, 12/15/25 to provide earlier visibility to Cardinal for routing on Tuesday, 12/16/25.
- New Years
- Adjust any ordering that typically falls on Monday, 12/29/25, Tuesday, 12/30/25, Wednesday, 12/31/25 & Thursday, 1/1/26 to be ordered by Monday, 12/22/25 to provide earlier visibility to Cardinal for routing on Tuesday, 12/23/25.
- MLK Day
- Adjust any ordering that typically falls on Thursday, 1/15/26, Friday, 1/16/26 and Monday, 1/19/26 to be ordered by Wednesday, 1/14/26 to provide earlier visibility to Cardinal for routing on Thursday, 1/15/26.
Communication Type:
Holiday Schedule
V#10801
- December 25
-
11/14/2025
Title: J&J V#10196
Details:
Hello,Please see the discontinuation notice from J&J V#10196. There has been no usage on these items in the last six months. Thanks!Discontinued
CodeC# SXMP1B426 264620 SXMP1B430 264621 SXMP1B431 264622 SXMP1B432 264623 SXPP1B115 264626 SXPP1B203 264571 SXPP1B204 #N/A SXPP1B205 264574 SXPP1B403 264578 SXPP1B423 264627 SXPP1B426 264604 SXPP1A417 #N/A SXPP1A437 #N/A Communication Type:
Discontinuation Notice
V#10196
-
11/13/2025
Title: Kimberly Clark V#10765
Details:
Hi, all.
Please see the attached allocation announcement from Kimberly Clark V#10765. The items below were added onto allocation and a customer listing is attached. Please contact Tricia Wentling if you have questions.
188783
01000
259339
01005
150005
02000
Communication Type:
Allocation Notification/Update
V#10765
-
11/13/2025
Title: B. Braun V#10508
Details:
Hello,Please see the allocation notice below and attached from B. Braun V#10508. The items below have been added onto allocation and a customer listing is attached. Please reach out to Heather Burkett if you have questions. Thanks!
B. Braun Concordance Nov Allocation Rpt 2025 - revised
CHS#
B. Braun Product Reorder Number
154977
2112361
287082
2112359
133552
412113
252907
2112365
Communication Type:
Allocation Notification/Update
V#10508
-
11/13/2025
Title: Kimberly Clark V#10765
Details:
Hello—
Below is only a summary about dates that Kimberly-Clark Professional V#1075 will be closed.
Please see the attachment for full details. This came from their portal.
Kimberly-Clark Professional® offices will be closed on Thursday, November 27 and Friday, November 28. Normal business will resume at 7:30 am EST on Monday, December 1.
- As outlined in the attachment, orders received between November 19 and November 26 will require additional lead time on the standard published lead times.
Kimberly-Clark Professional® offices will be closed Wednesday, December 24, and Thursday, December 25.
- Submit orders requesting shipment prior to year-end no later than 3:00 pm EST on Friday, December 12.
- Orders delivered by December 31, 2025, will receive current prices. All orders delivered on or after January 1, 2026, will receive the new into-stock price.
Communication Type:
Holiday Schedule
V#10765
-
11/13/2025
Title: Midmark V#10987
Details:
Hello,Please see the holiday shipping schedule from Midmark V#10987 below.Normal shipping will continue through December 23, 2025.December 24 – 25, 2025 – No shipping: Closed for the holidayDecember 26, 29 – 31, 2025 – Normal ShippingJanuary 1, 2026 – No shipping: Closed for the holidayJanuary 2, 5, 6, 2026 – Limited shipping due to inventory count: Shipping for “doctor down” parcel service
shipments onlyJanuary 7, 2026 – Normal shipping resumesCommunication Type:
Holiday Schedule
V#10987
-
11/13/2025
Title: ICU Medical V#10642
Details:
Hello,Please see the discontinuation notice below and attached from ICU Medical V#10642. There has been no usage on these items in the last six months.MFG#C#33-3300#N/A33-340012148833-3500#N/A33-350530942255-4700#N/A55-480018886355-6060#N/A55-6065#N/ACommunication Type:
Discontinuation Notice
V#10642
-
11/13/2025
Title: Teleflex V#10538
Details:
Hello,Please see the discontinuation notice below and attached from Teleflex V#10538. A usage file for the last six months is also attached. Please reach out to Heather Burkett if you have questions. Thanks!MFG#C#523835844415523835844415523700924035523800488627Communication Type:
Discontinuation Notice
V#10538
-
11/12/2025
Title: Roche Diagnostics V#10603
Details:
Hello-
Per the attached letter from Roche Diagnostics V#10603, they are experiencing production disruption with their supplier resulting in backorders of the following SKUs:
MFG #03617556001 (C#112931)
MFG #03260763602 (C#122433)
MFG #10467665001 (C#410769)
The letter states: We are working closely with our supplier to expedite the shipments of the reworked Urisys 1100 analyzers. The shipments are expected to arrive during the last week of November 2025. The Urisys 1100 analyzers will remain on allocation until January 2026, at which time, we expect to be back to normal supply levels.
This is the only usage in the past six months:
Account Manager ID
Account Manager Name
Location ID
Customer ID
Bill To Name
Customer Item ID
Mfg Stock Number
Mfg Code Description
VAI Item
Item Description
Sell UM
Unit Price
Order Qty
Ship Qty
Sales
1245
Rob Baldus-VA
WCST
K6802
WISCONSIN DEPT OF CORRECTIONS
03260763602
ROCHE DIAGNOSTICS CORPORATION
122433
KIT ANALYZER URISYS 1100 *DS*
EA
822.22
1
1
$822.22
Communication Type:
Backorder Notification/Update/Reports
V#10603
-
11/12/2025
Title: Solventum V#10296
Details:
Hello,Please see the discontinuation notice attached from Solventum V#10296. They are discontinuing their Vanish Varnish line and replacing it with Clinpro Clear. I have attached a usage file for the discontinued items.MFG #C#12149C27408612149L27410912149M27411312150C#N/A12150L27411412150M27411512151C#N/A12151L#N/A12151M#N/A12151CL#N/A12151X#N/ACommunication Type:
Discontinuation Notice
V#10296
-
11/11/2025
Title: Medline V#10605
Details:
Hello,Please see below and attached from Medline V#10605 regarding the discontinuation of their legacy oxygen mask line. I have attached a usage file for the discontinued items. Please reach out to Heather Burkett if you have questions. Thanks!Communication Type:
Discontinuation Notice
V#10605
-
11/11/2025
Title: Essity V#10272
Details:
Hello,Please see below and attached from Essity V#10272 regarding a packaging change. Products that are affected are Dry Comfort, Classic, Proskin Plus, and Proskin Gender Specific Underwear. The new bag design is the only change you will visibly notice with the product when receiving in from Essity. I have attached a usage file for the impacted items. Please reach out to Heather Burkett if you have further questions. Thanks!Communication Type:
Product Changes
V#10272
-
11/11/2025
Title: Molnlycke V#10008
Details:
Hi, everyone.
Molnlycke V#10008 is notifying us below and in the attached letter that HibiClens Packettes (SKU 57517) is on an extended backorder (this is C#134565).
Some key highlights of their letter:
- At this time, we do not have a confirmed date for when the Packettes will be healthy again. However, we are aiming for mid-February to start sending product again.
- We want to emphasize that all other Hibi products are unaffected by this delay, we have healthy stock, and are available for purchase.
- While we work diligently to resolve the issue, we’d like to offer alternative options to help meet your needs in the meantime:
- HibiClens 4oz Foam (SKU 57541) – this is C#284369
- HibiClens 4oz Liquid (SKU 57504) – this is C#266049
Communication Type:
Backorder Notification/Update/Reports
V#10008
-
11/11/2025
Title: Fortis V#13573
Details:
2025-EntrustOstomyAccessoryCatalog
2025-EntrustOstomyProductGuide
Entrust-AccessoriesCrossReference
Entrust-PouchCrossReferenceChart-2025
Hello Concordance Team,
Here is a message from Michelle Bliszack mbliszack@Fortismp.com: As your representative for Entrust Ostomy and Cymed pouch brands I am hoping that we will be able to build a relationship with you and your sales team.
Our goal is to offer the best pricing for Ostomy products so that you can build revenue and profitability in this tight category. By offering other brands rather than Hollister, Coloplast and Convatec will help you to achieve better outcomes for the category. The big three manufacturers all have their own providers so why continue to push their brands when they are just competing with you for business.
I am available when your team is ready to learn more about what Entrust and Cymed has to offer for Concordance Health Solutions. I would suggest that we set up a zoom call soon to discuss more about your initiatives for 2026 when it is convenient with you and your sales team.
Let’s partner together for 2026 and increase your revenue and profitability in Ostomy.
Communication Type:
Product Catalog
V#13573
-
11/11/2025
Title: Medela V#10262
Details:
Americas 2025 Year-End Shipping Schedule
Concordance Team,
Attached is Medela's recommended year end ordering schedule (V#10262).
Communication Type:
Holiday Schedule
V#10262
-
11/10/2025
Title: Nestle V#10183
Details:
Hello,
Attached is the Nestle Inventory Letter for this week.
1. Benecalorie
2. BOOST GLUCOSE CONTROL® MAX 30 g PROTEIN, Chocolate, 3 (4 x 11 fl
oz) carton
3. BOOST GLUCOSE CONTROL® MAX 30 g PROTEIN, Vanilla, 3 (4 x 11 fl oz)
Carton
4. BOOST®, Very Vanilla 24 x 8 fl oz bottle
5. NUTRISOURCE® FIBER, Unflavored Powder 75 x 4 g packets
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Communication Type:
Product Inventory Update
V#10183
-
11/10/2025
Title: Coloplast V#10581
Details:
Hello,Please see below and attached from Coloplast V#10581. This is an update on the discontinuation of the Freedom Clear line of male external catheters. I have included a usage file for the last six months as well. Please reach out to Tricia Wentling if you have questions. Thanks!I’m sending out the most recent Freedom® Clear estimated runout update. The chart is sorted first by hard discontinuation date, then by our current estimated runout date. You can find the latest runout dates in column ‘G.’ Please note that these dates may fluctuate based on demand, so keep this in mind when communicating with your accounts.
Below are the next codes estimated to run out this month:
Product code
Description
Type / length
Size (mm)
Qty / box
Estimated Runout
6230
Freedom Clear Adv.
Standard
28 mm
30
11/15/2025
6200
Freedom Clear Adv.
Standard
28 mm
100
11/28/2025
Communication Type:
Discontinuation Notice
V#10581
-
11/10/2025
Title: J&J V#10196
Details:
Hello,Please see the packaging update below and attached from J&J V#10196. I have attached a usage file for the impacted items.We are writing to provide you with advance notice of certain labeling and packaging changes to our Coated VICRYL™ (Polyglactin 910) Suture product line.
Please see the product table attached (Table #2 on pg.3) to this communication for the list of product codes (26) impacted by these labeling updates.
Please see the full customer letter attached.
There are no changes to any existing suture properties, such as handling or absorption. The SKUs remain the same as the current VICRYL sutures as listed in the product tables.
Communication Type:
Product Changes
V#10196
-
11/7/2025
Title: Abbott Nutrition V#10575
Details:
Dear Concordance Team,
We are sharing with all of our distributor partners the attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Communication Type:
Product Inventory Update
V#10575
-
11/7/2025
Title: Kenvue V#10860
Details:
Good morning,
Please see the attached communication from Kenvue V# 10860. I would like to draw attention to this part of the letter: “In terms of next steps, we expect to complete the transaction in the second half of 2026, subject to approval by shareholders of both companies, regulatory approvals and customary closing conditions. Until then, Kenvue and Kimberly-Clark will continue to operate independently as separate companies, and it will remain business as usual for all of us at Kenvue. As always, our team is focused on serving our customers and the needs of your business. There are no changes to our contracts and agreements or how we work with at this time.”
There is nothing for Concordance to do at this time. This is just sharing the future plans of these two companies
Communication Type:
Updated Supplier Information
V#10860
-
11/7/2025
Title: Tidi V#10260
Details:
Hello,Please see below and attached from Tidi V#10260. The equipment covers on the list attached are no longer on backorder. I have attached a usage report for the items that we carry. Please contact Heather Burkett if you have questions. Thanks!Communication Type:
Backorder Notification/Update/Reports
V10260
-
11/7/2025
Title: BD V#10125
Details:
Concordance Team
To help effectively plan your order fulfillment needs, enclosed is a copy of BD’s 2025 Holiday Schedule for the remainder of the calendar year.
Kindly note the following:
Thanksgiving Day (Thursday, 11/27)
- BD fully closed (no Customer Care coverage will be available)
- No rush orders and no deliveries will be processed
- FedEx operating under modified service
Day after Thanksgiving Day (Friday, 11/28)
- BD HUB1 DCs open; HUB2 DCs (Covington, GA and Sparks, MD) open
- BD Corporate Offices, NA Shared Service Center, El Paso, TX and Otay Mesa, CA DCs will be closed
- No rush orders will be processed on Friday, 11/28
Milk Run Planning (Thanksgiving Holiday)
- Locations that place orders on Monday to ship Thursday – Order as normal on Monday, 11/24 – Plan is to ship on Wednesday, 11/26 rather than Thursday, 11/27
- Customers that typically place orders on Thursday, 11/27 or Friday, 11/28 should plan to shift them to earlier in the week
- Locations that typically receive deliveries on Friday, 11/28 can expect deliveries on Monday, 12/1
Thank You,
Communication Type:
Holiday Schedule
V#10125
-
11/7/2025
Title: Cardinal V#10801
Details:
Hello,
Please see the information from Cardinal Health V#10801. CHS#110697 is on constraint per the email below. See below for alternatives.
The fresh news is that 22453 (CHS#110697) - will be constrained through December. There is a batch scheduled to be created at the end of October, so likely we won’t see these fill until early November when the shipment gets to the right warehouse.
If your customer is open to an alternative, we have a few options available out of R1AL (Atlanta RC) which are listed below:22853: radiolucent version of 22453-
Kendall™ Medi-Trace™ 850 Series Radiolucent Foam Electrodes | Cardinal Health
22455-: 5 pk version of 22453-
Kendall™ Medi-Trace™ 450 Foam Electrodes | Cardinal Health
31115796: diaphoretic, teardrop shape, 3 pk
Kendall™ Medi-Trace™ 530 Series Foam Electrodes | Cardinal Health
Communication Type:
Supply Disruption
V#10801
Communication Type:
Substitution Option
V#10801
-
11/7/2025
Title: Laerdal V#11506
Details:
Hello,
Please see the information below and attached from Laerdal V#11506. A usage file for the discontinued items is attached.
As part of our ongoing portfolio optimization efforts, we have streamlined and standardized the Pocket Mask product line to improve operational efficiency, simplify logistics, and better serve our markets.
Key changes include:
Multi-language catalog configurations: Previously, most catalog numbers were configured with a single language. We have now consolidated multiple languages into each product configuration. This reduces complexity and enhances usability across regions.
Aligned product availability:
o Generic versions:
· Polybag
· Hard Case (Yellow)
· Soft Pouch (Blue & Yellow)
(Available for both the standard Pocket Mask and the Pocket Mask with Oxygen Inlet)
- Generic versions for Pediatric Pocket Mask:
- Red & Black and Blue & Yellow Soft Pouch
- Discontinuation of gloves and wipes: Gloves and wipes have been removed from most configurations. This change extends shelf life to five years, eliminates the need for reworks, and simplifies inventory handling.
- Packaging simplification: We have discontinued single-pack and 100-pack formats. All products will now be supplied in 10-pack configurations only. Distributors may split these 10-packs into individual units for resale as needed.
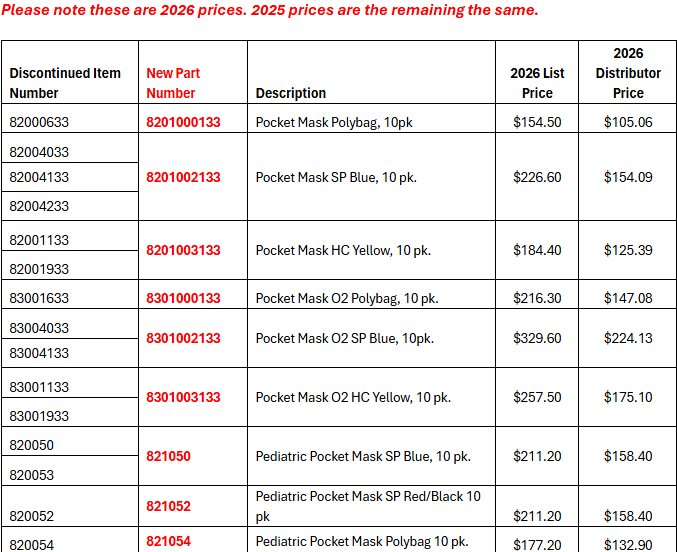
Communication Type:
Discontinuation Notice
V#11506
-
11/6/2025
Title: Tidi V#10260
Details:
Hello,Please see below and attached from Tidi V#10260.S#642948 #9210-250DSP discontinued and replaced with S#180238 #9210-250
S#642954 #9211-400 discontinued and replaced with S#180239 #9211-100
A usage file for the past six months is attached.
Please contact Heather Burkett if you have questions. Thanks!
Communication Type:
Discontinuation Notice
V#10260
-
11/6/2025
Title: Health-O-Meter V#10075
Details:
Hello,
Please see below and attached from Health-0-Meter V#10075.
I am reaching out to share a new product announcement for the Bridge Healthcare line that can be shared with your customers and sales team. For any communications I have also attached the images for these new items and a product information spreadsheet, here also is the website highlighting the product: https://bridgehcusa.com/flojac/
Please let me know if you need anything else from me regarding this information.
Bridge Healthcare by Pelstar Launches the Flojac® Fall Recovery Device
Bridge Healthcare by Pelstar is pleased to announce the launch of the Flojac® Fall Recovery Device, an innovative solution designed to safely and efficiently raise fallen patients from the floor. The Flojac is engineered to provide a controlled, low-stress recovery process for patients while reducing the physical strain and injury risk for caregivers. With a 1100lb capacity, the device is suitable for a wide range of patient populations and is quick to both inflate and deflate, allowing for streamlined use in urgent situations. Available as item # B-FLOJAC39, the Flojac is the latest addition to the Bridge Healthcare portfolio, complementing the company’s suite of safe patient handling and mobility solutions.


Communication Type:
New Product Launch
V#10075
-
11/6/2025
Title: Dermarite V#10174
Details:
Hello,Dermarite V#10174 has discontinued their skin care line. I have attached a list of discontinued items as well as a usage report for the last six months. Please reach out to Tricia Wentling if you have further questions. Thanks!Communication Type:
Discontinuation Notice
V#10174
-
11/6/2025
Title: Lynn Medical V#11186
Details:
Hello,
Please see below from Lynn Medical V#11186 regarding a special promotion. Please contact Tricia Wentling if you have questions.
I hope this email finds you well. We are starting to see flu, covid, strep, and RSV testing pick up again. Our manufacturer Lifesign extended their buy 5 boxes get a box free promotion through the end of the year. However, they are now allowing to mix and match amongst the different tests below.
Please reach out if you have any interest for our special pricing. Thanks!!
33225 Lifesign Covid-19/Flu A&B, 25 Tests/Bx
36025 Lifesign Rapid Status Flu A&B, CLIA waived for Swab Specimens, 25
34125 LifeSign LLC, Status Strep A Flip Cassette, CLIA Waived, 25 tests/bx (Item is Non-Returnable)
33125 Lifesign, Status RSV Rapid Test Kit Includes: (25) Individiaully Pouched Test Casettes, (25) Sterile Nasal Swabs, (25) Pre-filled Reagent Tubes with Dropper Tips, (1) Negative RSV Control Swab, (1) Positive RSV Control Swab, (1) Instructions for Use
Best Regards,
Ryan Milke
Regional Sales Manager, Lynn Medical248-560-4518 | lynnmed.com |
Communication Type:
End User Promo
V#11186
-
11/6/2025
Title: Airlife V#11179
Details:
Hello,
Please see the discontinuation below from Airlife V#11179. There have been no sales on this item.
S#237470 #R230P01 has been discontinued with no replacement. S#237470 has been placed in item inactivation.
Communication Type:
Discontinuation Notice
V#11179
-
11/6/2025
Title: Ansell V#11003
Details:
Hello,
Please see the ASC MTMC roster and Ansell ASC sell sheets below from Ansell V#11003.
Communication Type:
Sales Roster
V#11003
Communication Type:
Product Flyer
V#11003
-
11/5/2025
Title: BD V#10125
Details:
Hello,Please see the update from BD V#10125. Per Casey's email below, the items marked Temp de-prioritized have been updated to Temp Unavailable. The list of items and customer listing is attached. Please contact Heather Burkett if you have further questions. Thanks!MFGCHS#Notes10010454134789Temp De-prioritized2434-0007403201Temp De-prioritized11532269177978March 202610015012231831Temp De-prioritized2432-0007244946March 202624301-0007T231646Temp De-prioritized2465-0007208036March 202620350E733279March 202620027E218795March 202642933E217016Temp De-prioritized20350ET318957Temp De-prioritized10013902223449Temp De-prioritizedCommunication Type:
Temp Unavailable
V#10125
-
11/5/2025
Title: Baxter V#10797
Details:
Hello,Per the attached email from Baxter V#10797, S#110970 (Mfg #H938175) and S#112511 (Mfg #H938173) are no longer on allocation. I have attached a usage file for the last six months. Thank you!
Communication Type:
Allocation Notification/Update
V#10797
-
11/5/2025
Title: Welch Allyn V#10834
Details:
Hello,Please see the attached from Welch Allyn V#10834 on the discontinuation of the Welch Allyn Eco-Cuff. I have attached a usage file for the last six months. Please contact Tricia Wentling if you have further questions. Thanks!C#MFG#201732ECOCUFF-12201737ECOCUFF-11201740ECOCUFF-10201743ECOCUFF-09Communication Type:
Discontinuation Notice
V#10834
-
11/4/2025
Title: GOJO V#10354
Details:
Hello,
Please see below and attached from GOJO V#10354 regarding their upcoming holiday schedule.
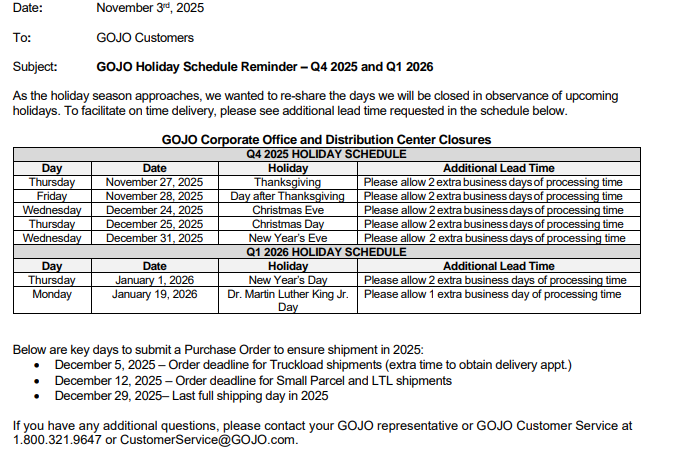
Communication Type:
Holiday Schedule
V#10354
-
11/4/2025
Title: BD V#10125
Details:
Hello,Please see the attached from BD V#10125. This provides an update regarding adjusted Hypodermics benchmark allocation to end customers through November 2025. A customer listing is also attached and new percentages are also attached.
309628 312686 110% 309659 173722 90% 309657 173729 Not on allocation 309646 166243 110% 309647 166787 120% 302995 240059 Not on allocation 302830 196669 Not on allocation 302832 196672 Not on allocation 309653 299720 Not on allocation 305062 270165 Not on allocation 305064 531087 Not on allocation Communication Type:
Allocation Notification/Update
V#10125
-
11/4/2025
Title: Laerdal V#11506
Details:
Hello,
Please see below and attached from Lardal V#11506. The attached list of items is being discontinued. I have included a usage file for the last six months. Please contact Tricia Wentling if you have further questions. Thanks!
Communication Type:
Discontinuation Notice
V#11506
-
11/4/2025
Title: Medtronic V#10255
Details:
Hello,Please see the updated discontinuation notice from Medtronic V#10255. I have attached a usage file for the last six months. Thanks!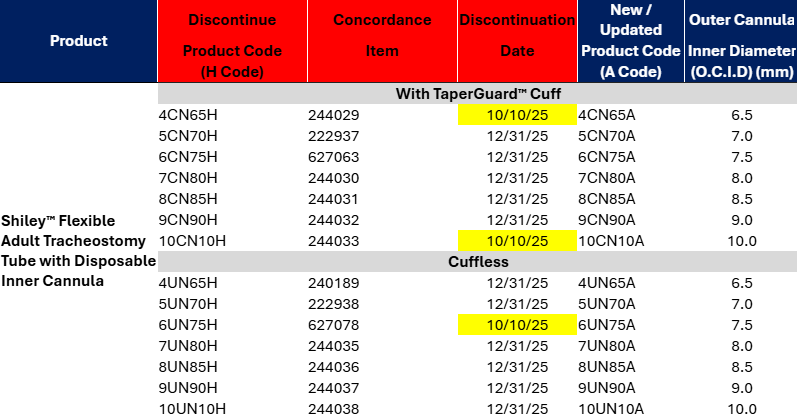
Communication Type:
Discontinuation Notice
V#10255
-
11/3/2025
Title: Fortis Medical Products V#13573
Details:
Hello,
Please see the email below and flyer attached about a new product offering from Fortis V#13573.
I thought I would share with you one of our newest products. It is not Ostomy related but it is a new wound wash – DermaCleenz. I have attached a flyer for more information about the product. If you would please share this with your sales team to see if they have any customers that are looking for a low cost wound wash product.
Communication Type:
New Product Launch
V#13573
-
10/31/2025
Title: Laerdal V#11506
Details:
Hello,Please see below and attached for a discontinuation notice from Laerdal V#11506. I have attached a usage file for the last six months. Thanks!Number
Description
Available Stock till it runs out
C#980100
Stifneck Baby
871
754655980200
Stifneck Pediatric
3,698
754663980300
Stifneck
6,432
754671980400
Stifneck Short
1,154
305071980500
Stifneck Regular
1,750
305072980600
Stifneck Tall
333
NA980700
Stifneck Carry Bag
284
770821Communication Type:
Discontinuation Notice
V#11506
-
10/31/2025
Title: Seca V#10116
Details:
Hello,
Please see the information below and attached on a new scale offered by Seca V#10116.
I’m following up from earlier this summer about our new medical body composition analyzer, seca Alpha mBCA, designed for medical weight loss programs looking for an affordable, compact body composition solution, to see if we can get a Concordance SKU assigned. Since it rolled-out a few months ago it’s been selling like gangbusters mainly to weight loss and wellness programs (also fitness and sports markets). To get things rolling I’ve attached the description, details and 2025 pricing.
There’s a lot of buzz around this product - clinicians are seeking to monitor changes in body composition when patients are taking the new weight loss medications among other applications of the technology.
Communication Type:
Product Flyer
V#10116
-
10/31/2025
Title: BD V#10125
Details:
Hello,Please see below and attached regarding an item on backorder from BD V#10125. I have attached a usage file for the last 6 months.Due to a production delay at the manufacturing site, 405211 (C# 940452) will be on a prolonged backorder until January 2026.Please see below substitute options.BD Catalog Number BD Catalog Description405148 NEEDLE SPINAL S/SU 22GA 5IN QUINCKE405149 NEEDLE SPINAL S/SU 22GA 7IN QUINCKE408360 NEEDLE SPINAL S/SU 18GA 6IN QUINCKECommunication Type:
Backorder Notification/Update/Reports
V#10125
-
10/30/2025
Title: Medline V#10605
Details:
Hello,
Per the email below from Julie @ Medline, S#286877 (Mfg #SYRSI193292) and S#278114 (Mfg #SYRSI101292) are being discontinued. A pricing request has been submitted to Medline for Mfg #ULT193292. Once I receive pricing, I will address S#286877. S#278114 has already been placed into item inactivation and should inactivate overnight. The replacement for that item is not being set up due to lack of sales. Please let me know if you have any questions. Thank you!
A usage file for the discontinued items is attached.
Due to a vendor disruption, Medline’s Safety Insulin Syringe will be discontinued and replaced by the UltiCare Safety Insulin Syringe, effective 11/15/2025. Please read below for important information about this change. Our distribution partners will not be allocated product until this transition is complete.
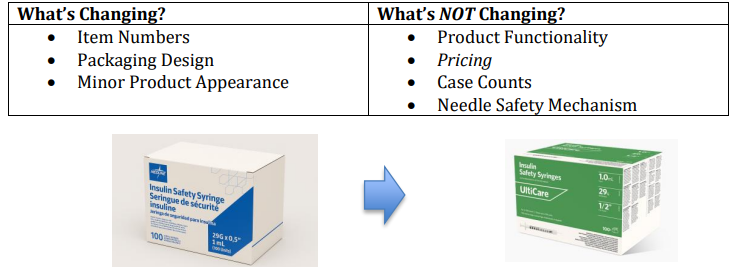
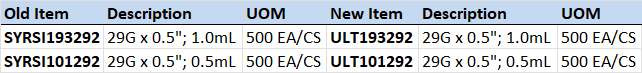
Our distribution partners will not be allocated product until this transition is complete.
Timing of this transition will vary based on item and local branch stock. Please see below for expected transition dates:SYRSI193292, 29G x 0.5"; 1.0mL - 11/17/2025
SYRSI101292, 29G x 0.5"; 0.5mL - 12/29/2025
Communication Type:
Discontinuation Notice
V#10605
-
10/30/2025
Title: Medi USA V#10848
Details:
Hello,
Please see the end of year shipping deadlines below from Medi USA V#10848.
The end of the year is quickly approaching! To help ensure your patients and customers receive their custom medi® products before the holidays, please review the production order deadlines below for shipments by December 31, 2025.
To meet these timelines, please make sure all custom orders are 100% complete and accepted into production by the cutoff dates listed below.
Custom Order Deadlines:
• Custom flat-knit trend colors – orders received by Tuesday, December 2, 2025
• Custom flat-knit standard colors – orders received by Tuesday, December 9, 2025
• Custom circular-knit – orders received by Wednesday, December 17, 2025
• Custom circaid® – orders received by Friday, December 26, 2025We appreciate your partnership and continued trust in medi®. Our team is here to support you through the busy holiday season and to help you finish the year strong.
Communication Type:
Holiday Schedule
V#10848
-
10/30/2025
Title: Molnlycke V#10008
Details:
Hello,Please see the attached holiday schedule for Molnlycke V#10008. Thanks!As we approach the holiday season, Molnlycke is proactively planning our operations to ensure continued service excellence to you and our mutual end-user customers. Please be aware that shortages of drivers during the holiday weeks could create disruption, and with that we ask you to pull forward orders in advance to help ensure timely deliveries and to minimize service disruptions.
Please take note of the following holiday closures and associated order deadlines:
- Thanksgiving Closure: Molnlycke will be closed on November 27th and 28th, 2025. To avoid any disruption in your normal weekly inventory, we kindly request that all orders be placed by Friday, November 21st.
- Christmas & New Year Closure: Molnlycke will be closed on December 24th, 25th, and January 1st, 2026. To avoid any disruption in your normal weekly inventory, we ask that all normal weekly order volumes for December 22nd through January 1st be placed between December 9th and December 19th.
Please allow an additional 3 days to your expected delivery date to accommodate increased processing time.
If you have any questions regarding Molnlycke’s holiday schedule or need support with planning, please contact our Customer Experience Team:
Email: customer.orders@molnlycke.com
Phone: 1-800-843-8497.
Attached is our official Distribution Notification for you to share within your organization and save to your files.
Thank you for your continued partnership and support!
Communication Type:
Holiday Schedule
V#10008
-
10/29/2025
Title: Moldex V#10503
Details:
Hello,Please see below and attached from Moldex V#10503 about a product update with regard to a new warning label. I have attached a usage file for the impacted items. Thanks!
Dear Valued Customer,
We want to provide you with an important update regarding our products. Due to changes in Prop. 65 regulations, effective January 3rd our products will be labeled with the required warning language seen below. Please share this communication and linked product list with relevant contacts.
Warning: Risk of cancer from exposure to vinyl acetate.
Communication Type:
Label Changes
V#10503
-
10/29/2025
Title: Teleflex V#10538
Details:
Hello,Please see the update from Teleflex V#10538 below. Thanks!We understand that you may have experienced recent delays in product shipments and other shipping-related issues. In this regard, we are in the process of completing an upgrade to our warehouse logistics system at our North American Distribution Center (NADC). This upgrade is a necessary step to further fortify long-term business continuity systems and, importantly, to enhance how we serve you, our valued customer.
While this transition is designed to enhance future service, we understand the essential nature of the products we supply and apologize for any impact this may have had on your operations. Please know that resolving these issues remains our top priority.
Current Status:- Parcel orders (<150 lbs) received by 4 PM ET are processed and shipped within 24 hours.
- LTL orders (>150 lbs) are processed and shipped within 48 hours of receipt.
- Expedited orders (overnight/2-day) received by 4 PM ET are shipped same day.
- Tracking information and documentation inconsistencies are actively being resolved.
- Additional resources are in place to maintain consistent performance.
We are committed to restoring full confidence in our delivery capabilities and will continue to provide updates. If you require urgent support, including access to packing lists or shipment details, please contact Customer Service at cs@teleflex.com.
Thank you for your continued understanding and partnership.
Sincerely,
Teleflex Customer Experience TeamCommunication Type:
Updated Supplier Information
V#10538
-
10/29/2025
Title: Jant V#10420
Details:
Hello All,
Please see discontinued item CHS#177874 from JantV# 10420, with replacement listed. A usage file is attached.
S#177874 (Mfg #CS625) has been discontinued and placed into item inactivation. It should inactivate overnight and direct to S#428369 (Mfg #CS425).
Please note that we have recently discontinued the IFOBT item CS625. To seamlessly meet your needs, we are moving forward with our ValuPak IFOBT, CS425, an already established Jant product on your 2025 price list. Details:
CS425: $31.25/box (25 tests) - Accutest® ValuPak™ Immunological Fecal Occult Blood Test. Includes 25 Single-Well Test Devices, 25 collection tubes w/buffer reagent & instructional insert.
For information: https://www.jantdx.com/product/accutest-valupak-immunological-fecal-occult-blood-ifobt-test-single-sample-test/
If you would like to sample the ValuPak IFOBT kit, please respond to this email.
Thank you,
Tiffany
Tiffany Mathias
818.203.2410
Regional Sales ManagerCommunication Type:
Discontinuation Notice
V#10420
-
10/28/2025
Title: Laborie V#11390
Details:
Hello,Please see below and attached from Laborie V#11390.VAC6000S (C#317480) is currently unavailable. Suggested replacement is VAC-6000M (C#317477). You can order the VAC-6000M to fill your orders for the VAC-6000S at the price point of the VAC-6000S. I have attached a usage file for the last six months.
Alternative Item
Unavailable
Alternative
VAC-6000S
Kiwi® ProCup®
VAC-6000M
Kiwi® OmniCup®
Communication Type:
Temp Unavailable
V#11390
-
10/24/2025
Title: Roche V#10603
Details:
Concordance Team,
See the attached letter from Roche Diagnostics V#10603 regarding their holiday and year-end shipping schedule.
Thanksgiving
- Roche Diagnostics will be closed on Thursday, November 27th and Friday, November 28th.
- Standing Orders scheduled to deliver between November 24 – December 1 may be processed early in an effort to deliver before the Thanksgiving holiday when possible.
Year End Holidays
- Roche Diagnostics will be closed on Wednesday, December 24th, Thursday, December
25th, and Friday, December 26th, as well as Wednesday, December 31st and Thursday,
January 1st (2026). - Customers are strongly encouraged to place orders before Wednesday, December 10th to
ensure delivery before the year end holidays. - Standing Orders scheduled to deliver during the last two weeks of the year may be
processed early in an effort to arrive and deliver before year end when possible.
Communication Type:
Holiday Schedule
V#10603
-
10/24/2025
Title: Stryker V#10912
Details:
Concordance Team,
Please see the attached announcement from Stryker about their new product offerings.

Communication Type:
New Product Launch
V#10912
-
10/23/2025
Title: Solventum V#10296
Details:
Hello,Please see below and attached regarding an allocation from Solventum V#10296. An updated usage file is attached.CHS#106463 (mfg#9200) - 50% allocation thru 12/31/25.CHS#177643 (mfg#9216) - Backordered until February 28, 2026.CHS#105809 (mfg#9222) - Backordered until February 28, 2026.Please reach out to Tricia Wentling if you have further questions.Communication Type:
Allocation Notification/Update
V#10296
-
10/23/2025
Title: Teleflex V#10538
Details:
Hello,Please see below and attached letter regarding discontinued items from Teleflex V#10538. I have attached a usage file for the last six months.These items have been placed in item inactivation:
S#913228 disc and suggested sub S#235168S#237782 disc and suggested sub S#428081
S#329517 disc and replacement not set up
S#365079 disc and replacement not set up
S#237784 disc and replacement not set up
S#237815 disc and suggested sub S#428082
S#237820 disc and replacement not set up
S#237819 disc and replacement not set up
S#237817 disc and replacement not set up
S#234467 disc and replacement not set up
S#243155 disc and suggested sub S#36020
S#281148 disc no replacement
S#274618 disc and replacement not set up
S#191482 disc and replacement not set up
S#284470 disc and replacement not set up
S#281142 disc and replacement not set up
S#191483 disc and replacement not set up
S#234471 disc and replacement not set up
S#234468 disc and replacement not set up
S#210255 disc and replacement not set up
S#281144 disc and replacement not set up
S#234472 disc and replacement not set up
S#207145 disc and replacement not set up
S#189277 disc and replacement not set up
S#228809 disc and replacement not set up
S#363272 disc and replacement not set up
S#189278 disc and replacement not set up
S#188234 disc and replacement not set up
S#276167 disc and replacement not set up
Communication Type:
Discontinuation Notice
V#10538
-
10/23/2025
Title: BD V#10125
Details:
Hello,Please see below regarding a new Veritor SKU from BD.Per the attached customer letter from BD V#10125, S#326088 (Mfg #256082) has been discontinued and replaced by S#428181 (Mfg #256092). S#326088 has been placed into item inactivation. I have attached a usage file and customer listing for the discontinued item. Thanks!Communication Type:
Discontinuation Notice
V#10125
-
10/23/2025
Title: Sage V#10229
Details:
Hello,The following items from Sage V#10229 have been added onto allocation at 150% and a customer listing is attached.
- Sage item #9705 CHS#110286
- Sage item #9717 CHS#417787
More information from their email below:- For current Sage CHG customers
- Because another manufacturer is having supply issues, we want to ensure that current Sage customers are not impacted
- Therefore, please put current Sage customers on 150% allocation to protect the current inventory in your channel
- For non-Sage CHG customers who want to use Sage CHG as a substitute
- Sage wants to support this demand
- Sage requires visibility into the account name and projected usage to ensure the appropriate production and supply levels are in place
- Please send an email to Teresa Tofel and me, with the account name, estimated forecast, and Concordance DC location for insight before the PO is placed
- Sage will not accept returns for CHG that was ordered as a sub product as CHG is a drug code and deemed unsaleable upon return
- Customers will need to bleed through the Sage CHG they purchased before returning to their regular manufacturer
-
Sage prefers shipping increased volume directly to your DCs to avoid the need for a drop ship PO
Please reach out to Tricia Wentling if you have further questions. Thanks!Communication Type:
Allocation Notification/Update
V#10229
-
10/23/2025
Title: Abbott Nutrition V#10575
Details:
Dear Concordance Team,
We are sharing with all of our distributor partners the attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Communication Type:
Product Inventory Update
V#10575
-
10/23/2025
Title: Moog V#10244
Details:
Hello,Please see the attached discontinued items and customer letter from Moog V#10244. A usage file for the last six months is also attached. Thanks!"340" Product Codes being discontinued CHS# 340-4114 213283 340-4126 213287 340-4128 213292 340-4128-V 340-4130 213296 340-4130-V 142693 340-4134 259250 340-4144 173632 340-4165 213312 340-4166 213304 340-4168 820297 340-4173 340-4174 Communication Type:
Discontinuation Notice
V#10244
-
10/21/2025
Title: Medline V#10605
Details:
Hello,Please see below and attached from Medline V#10605 regarding two items being put on allocation. The items included are as follows:285633 MSC096CHG379063 MSC099CHGA customer listing is attached. Please see Ashley's email below for more information:The ReadyPrep CHG® Wipes are experiencing a supply disruption. Below has key details for distribution and customers.
Allocation Update
- Distributors will receive no allocation of CHG wipes and all orders should be cancelled.
- The plan is to take the rest of the business direct in the interim.
- All customer questions should be directed to the Medline Sales Representative
Supply Chain Disruption
- Current open POs will not be filled and should be cancelled.
- Attached is the official letter that can be shared with customers
Product Safety
- Any CHG wipes currently in inventory or at Medline branches are safe and effective for use (see attached letter).
Impact Scope & Substitutions
- The disruption only affects SKUs MSC099CHG and MSC096CHG.
- Substitutes are available:
- Sage CHG Cloths:
- MSC099CHG → SGE9717 (6 wipes/pk, scented, same CHG dosage)
- MSC096CHG → SGE9705 (2 wipes/pk)
- Medline Alternatives:
- MDS098710 – Dyna-Hex 4% CHG Liquid Bottle
-
ULTRASOFT1013 – Ultrasoft Absorbent Dry Cleansing Wipes
- Sage CHG Cloths:
Please contact Heather Burkett if you have further questions. Thanks!Communication Type:
Allocation Notification/Update
V#10605
-
10/17/2025
Title: Hollister V#10901
Details:
Hello,Per the attached email, the following Hollister Ostomy V#10901 products will be discontinued effective December 31st, 2025, or until inventory is depleted. The recommended substitute is included in the table below. A usage file for the last six months is also attached. Please reach out to Mary Reinhart if you have further questions. Thanks!
Communication Type:
Discontinuation Notice
V#10901
-
10/17/2025
Title: Abbott Nutrition V#10575
Details:
Dear Concordance Team,
We are sharing with all of our distributor partners the attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Communication Type:
Product Inventory Update
V#10575
-
10/17/2025
Title: Nestle V#10183
Details:
Concordance Team,
Attached is the Nestle inventory letter for next week. Have a great weekend
1. Benecalorie was added to the report
2. BOOST GLUCOSE CONTROL® MAX 30 g PROTEIN, Chocolate, 3 (4 x 11 fl
oz) carton – Retail was added to the report
3. BOOST GLUCOSE CONTROL® MAX 30 g PROTEIN, Vanilla, 3 (4 x 11 fl oz)
carton – Retail was added to the report
4. COMPLEAT® ORIGINAL 1.5, Fruit Medley 24 x 250 mL carton was added
5. Thickenup Clear 4.4 oz canister is still on the report
6. COMPLEAT® ORIGINAL 1.0, Spike Right® PLUS 6 x 1000 mL UltraPak® bags
COMPLEAT® PEDIATRIC STANDARD 1.4 Cal, Vanilla 24 x 250 mL carton was added
7. COMPLEAT® STANDARD 1.4 Cal SpikeRight® PLUS 6 x 1000 mL UltraPak®
Bags
8. COMPLEAT® PEPTIDE 1.5 Cal, SpikeRight® PLUS 6 x 1000 mL UltraPak®
bags
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Communication Type:
Product Inventory Update
V#10183
-
10/16/2025
Title: SC Johnson V#12219
Details:
Concordance Team,
Please see attached from SC Johnson V#12219 regarding their new hand sanitizing products. According to Don's email, the competition doesn't offer anything similar. This may be an opportunity to target customers and then get the SC Johnson sales team involved. Please reach out to Tricia Wentling if you have any questions. Thanks!
Alcare® Instant Foaming 72% Alcohol Sanitizer
Communication Type:
New Product Launch
V#12219
-
10/13/2025
Title: Nestle V#10183
Details:
Concordance Team,
Attaached is the Nestle Inventory letter for this week. Please contact Mary Reinhart if you have any questions.
1. Benecalorie was added to the report
2. Boost Chocolate is still on the report
3. BOOST GLUCOSE CONTROL® MAX 30 g PROTEIN, Chocolate, 3 (4 x 11 fl
oz) carton – Retail was added to the report
4. BOOST GLUCOSE CONTROL® MAX 30 g PROTEIN, Vanilla, 3 (4 x 11 fl oz)
carton – Retail was added to the report
5. Compleat Pediatric Peptide 1.0 250 ml was added tp the report
6. Thickenup Clear 4.4 oz canister is still on the report
7. COMPLEAT® ORIGINAL 1.0, Spike Right® PLUS 6 x 1000 mL UltraPak® bags was added
8. COMPLEAT® STANDARD 1.4 Cal SpikeRight® PLUS 6 x 1000 mL UltraPak®
Bags
9. PEPTAMEN® 1.5 with PREBIO¹™, SpikeRight® PLUS 6 x 1000 mL UltraPak®
bags
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Communication Type:
Product Inventory Update
V#10183
-
10/13/2025
Title: Attends V#10055
Details:
Hello,Please see below from Attends V#10055 about 3 items being discontinued as well as their replacements. I have attached a usage file for the discontinued items.The ALI-UW30 in now Officially out, and please note: To Discontinue to the 3 Items below due to lack of demand by Concordance customers.
CURRENT NEW TRANSITION DATE CHS #
- ALI-UW20 => ALI-UW20S 3/12/2026 285554
- ALI-UW30 => ALI-UW30S 10/9/2025 285556
- ALI-UW40 => ALI-UW40S 10/21/2025 285557
Communication Type:
Discontinuation Notice
V#10055
-
10/13/2025
Title: Coloplast V#10581
Details:
Hello,Please see below and attached from Coloplast V#10581. This is an update on the discontinuation of the Freedom Clear line of male external catheters. I have included a usage file for the last six months as well. Please reach out to Tricia Wentling if you have questions. Thanks!I’m sending out the most recent Freedom® Clear estimated runout update. The chart is sorted by hard discontinuation date and then our current estimated runout date. You can find the latest runout date in column ‘G’ – note these dates may fluctuate with demand, so please be mindful in your communication.
A few items to bring attention to:
- In the last week, we have depleted inventory of code 5110.
- The next codes estimated to runout, in October, are included below.
Product code
Description
Type / length
Size (mm)
Qty / box
Estimated Runout
5100
Freedom Clear
Standard
23 mm
100
10/5/2025
5500
Freedom Clear
Standard
40 mm
100
10/11/2025
6300
Freedom Clear Adv.
Standard
31 mm
100
10/21/2025
Communication Type:
Discontinuation Notice
V#10851
-
10/10/2025
Title: Derron Surgical Instruments V#12478
Details:
Concordance Team,
Please see below from Derron Surgical V#12478 regarding a price increase for 2026. Please reach out to Mary Reinhart if you have questions.
We greatly value your trust in DERRON Surgical Instruments as a key business partner. Thank you for your continued partnership. Our priority remains, to supply surgical instruments of the highest quality and the best service to our customers.
As you are aware, ongoing shifts in the global economic situation, including increases in tariff rates, have created significant challenges across the supply chain. To maintain high standards of quality and inventory reliability, DERRON Instruments must unfortunately share a portion of these tariff-related costs with our customers.
Per updated tariff rates from the United States Customers and Border Protection (US CBP) as of August 1, 2025, products from the European Union (i.e., Germany) will have a 15% tariff rate. These are updates to the initial US CBP baseline 10% tariff rate from April.
Our 2026 Price list will reflect a 10% increase. We are facing growing challenges: rising costs of manufacturing, international transportation costs and the Tariff Recovery Surcharge. Since April 1, 2025 we have absorbed this expense.
We will continue to keep our product pricing as stable as possible and provide competitive industry pricing, high quality products, and high inventory availability of the 7,000+ SKU’s we that we can provide.
We understand that these charges may cause an inconvenience and we appreciate your understanding. Should you have any questions, please contact me directly for information on related trade policy.
We thank you for your understanding and look forward to continuing our successful cooperation with you.
Best Regards,
Greg Derron
DERRON Surgical Instruments, Inc
941.918.8852
Communication Type:
Tarriff
V#12478
Communication Type:
pricing_changes
V#12478
-
10/10/2025
Title: Abbott Nutrition V#10575
Details:
Dear Concordance Team,
We are sharing with all of our distributor partners the attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Communication Type:
Product Inventory Update
V#10575
-
10/10/2025
Title: BD V#10125
Details:
Good afternoon!
Please see attached allocation updates from BD, V#10125. The items listed below have been removed. Attached is an updated customer listing.
Please reach out to Heather Burkett if you have any questions. Thanks!
306413
Flush
239866
306414
Flush
237802
306423
Flush
264391
306424
Flush
227053
306493
Flush
345787
306495
Flush
345784
306499
Flush
284361
306544
Flush
149010
306545
Flush
308370
306546
Flush
886754
306547
Flush
158576
306553
Flush
146045
30654678
Flush
295792
30654778
Flush
329283
Communication Type:
Allocation Notification/Update
V#10125
-
10/10/2025
Title: Attends V#10055
Details:
Hello,
Please see the new product offering below from Attends V#10055

Coming Soon... Sleep Comfort ™ Premium Overnight Underwear
Our NEW Premium Overnight Underwear is designed for extended hours of sleep, ensuring comfort and dryness from dusk till dawn for up to 12 hours.
Retail customers are seeking high-quality overnight products they can trust, and now with Sleep Comfort™, you can provide that to them at an affordable MAP protected price (shown below).
Available at the end of October 2025.

Our NEW Sleep Comfort ™ Premium Overnight Underwear delivers exceptional performance at an affordable cost, making it an easy addition to your product lineup.
Why Sleep Comfort ™ will work for you:
- Up to 12 hours of dryness – fewer product changes mean more satisfied users.
- Comfortable, flexible fit – designed for active sleepers who move throughout the night.
- Dry-Lock® Containment Core quickly locks away liquid and ConfidenceCuff™ helps prevent leaks around the legs
- Strong value at cost – Quality your customers expect, margin your business needs.
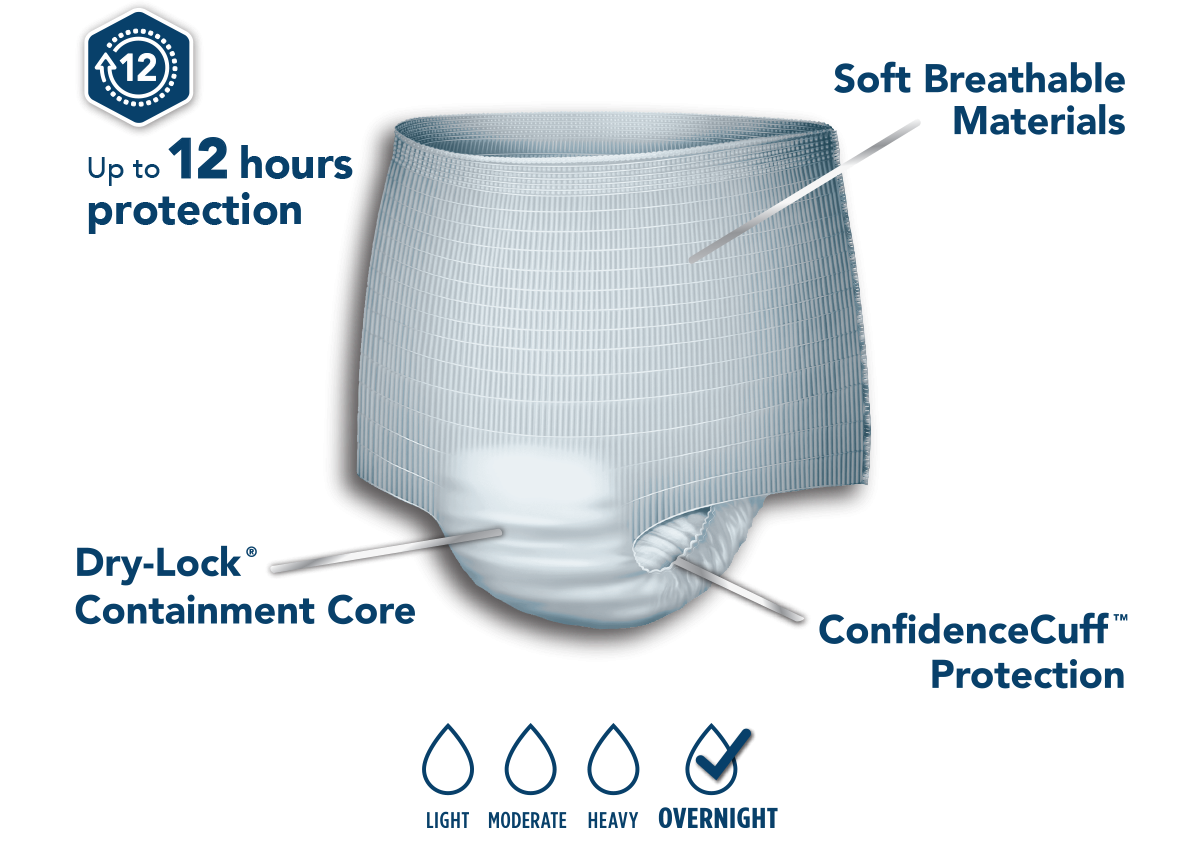
With Sleep Comfort™, you’ll strengthen trust, boost loyalty,
and create new opportunities for growth in your incontinence category.
Communication Type:
New Product Launch
V#10055
-
10/10/2025
Title: Marketlab V#11935
Details:
Hello All,
Please see END USER Promo from Bowman(now under Marketlab V#11935). This is a hygiene station that coincides with cold and flu season.
All details are attached and below to share with your customers. Any questions, please reach out to Bob Kelly, contact info below or Mary Reinhart.
I wanted to make sure you have the details on our Bowman® Hygiene Station Promotion running this cold and flu season. It’s a great opportunity for your customers to prepare their facilities and get rewarded at the same time.
Here’s how it works:
When your customers purchase a qualifying hygiene station between October 1, 2025 - March 31, 2026, they can redeem a $25.00 rebate OR 2 free glove box holders per item purchased.
This aligns with our campaign theme, Prevention Starts at the Station—encouraging facilities to place hygiene stations in high-traffic areas to help stop the spread before it starts.
Promo Details:
1. Qualifying products: BD101-0012, BD105-0012, KS022-0012
2. Purchase window: October 1, 2025 – March 31, 2026
3. Customer reward: choice of $25 rebate OR 2 free glove box holders per item purchased
Please have your sales team share the PROMO and REDEMPTION details with your customers here: Stop The Spread Promo
Let me know if you have any questions or if I can help you drive this promotion with any of your customers.
Best
Bob
Bob Kelly
Senior Strategic Account Manager
860.550.1240Communication Type:
End User Promo
V#11935
-
10/10/2025
Title: S2S V#10006
Details:
Hello All,
Please see notification from S2S V#10006, and REMOVAL of ALLOCATION. Please see your customer listing and attached notification to share with your customers. The items below have been removed from allocation:
6520 641325
676108 326889
7601 326894
7603 326897
7604 326900
7608 326909
7623 326911
7630 326774
Please contact Mary Reinhart if you have questions. Thanks!
Communication Type:
Allocation Notification/Update
V#10006
-
10/9/2025
Title: Molnlycke V#10008
Details:
Concordance Team,
Please see below and attached from Molnlycke V#10008 regarding items that have been discontinued. I have attached a usage file for the past six months. Please reach out to Tricia Wentling if you have further questions. Thanks!On behalf of Mölnlycke and the Hibi Antiseptics Business Area, I want to express our sincere appreciation for the valuable partnership we have established over many years. Your commitment to providing quality healthcare solutions to end users has been instrumental to our mutual success.After the careful strategic review of our previous larger sized 32oz and 1 Gal bottle discontinuation, we have made the decision to discontinue these SKUs as well:
Previously Discontinued:
57532 Hibi 32oz Bottle
57591 Hibi 1 Gal Bottle
Now Discontinued:
59902 Hibi 32oz Hand Pump
59903 Hibi 1-gallon Hand Pump
59904 Hibi Pump Assembly
59905 Hibi Foot Pedal
59994 Hibi Foot Wall Dispenser
59996 Hibi Hand Wall Dispenser
Communication Type:
Discontinuation Notice
V#10008
-
10/9/2025
Title: Audit Microcontrols V#12466
Details:
Concordance Team,
Please see below and attached from Audit Microcontrols V#12466 about their new product offering.
GREAT NEWS….we just launched a new product, K829M-10 Linearity FD Urine Chemistry for Beckman AU, and wanted to be sure you were aware.
I have attached a copy of the sell sheet, IFU and price list for your reference.
If you have any questions, please do not hesitate to reach out.
Jennie Marshall
Operations Manager
Email: jmarshall@auditmicro.com
Fax: 706.485.2123
Communication Type:
New Product Launch
V#12466
-
10/9/2025
Title: Fresenius V#10068
Details:
Concordance Team,
Please see below and attached from Fresenius V#10068.
My primary reason for reaching out to you is to communicate that we are continuing the process of transitioning from our PureFlow B dialysate products to the new Bicarby product line. Starting October 1st, we will no longer accept orders for PureFlow B RFP-404. From that date forward, we will be moving all of this volume to the replacement SKU, Bicarby RFP-404-G; these products have the same formulation. Attached is a guide for the Bicarby RFP-404-G transition, including a section with product-specific details. We are making sure that you have this information in advance of our shared customers. With that, on Monday, August 25th, our field team will share communication outlining these details with both your local representative and the relevant hospital contacts. They will also be providing the product transition guide to help streamline the transition process locally.
Based in our records, this is only pertinent to one Concordance customer: Union Hospital (Terre Haute, IN).
Please reach out to Heather Burkett if you have questions.
Communication Type:
Discontinuation Notice
V#10068
Communication Type:
Product Changes
V#10068
-
10/9/2025
Title: NDC V#10030
Details:

Provider Flyers from
Top Manufacturer Partners
Flyers to Support Your Marketing EffortsFeatured Vendors: 
View all Provider Flyers 


Ansell
Q4 PromoBaxter
Smart TechnologyBD
Extended PromoDownload Download Download 


BD
RSVClensTech
Wound IrrigationClorox
Screen Plus WipesDownload Download Download 


Dukal
Adhesive BandgesDuracell
Procell in HealthcareGOJO
ES10Download Download Download 


Graham Medical
Q4 PromoHealth o meter
RollboardMedtronic
ASCDownload Download Download 


Metrex
HPMidmark
Better BPMidmark
CabinetryDownload Download Download 


Midmark
Digital ECGMidmark
HEINEMidmark
IP SolutionsDownload Download Download 


Midmark
Optimize WorkflowMidmark
Procedure ChairMidmark
WorkstationsDownload Download Download 


O&M Halyard
Law Black GlovesO&M Halyard
Tattoo Black GlovesPDI
Floorstand PromoDownload Download Download 


PDI
Prevantics Cross ReferencePDI
Premium Dental PackagePDI
Premium LTC PackageDownload Download Download 


PDI
Premium ASC PackagePDI
Prevantics FamilyPDI
Product CatalogDownload Download Download 


PDI
Sani-Cloth OverviewPDI
Sani-HandsPDI
Sani-HP1 BrochureDownload Download Download 


SEKISUI
Q4 PromoSmith+Nephew
ALLEVYN Ag+Smith+Nephew
ALLEVYN FamilyDownload Download Download 


Smith+Nephew
PICO 7Smith+Nephew
PICO 14Smith+Nephew
PICO FamilyDownload Download Download 


Smith+Nephew
PICO Case StudySmith+Nephew
Injury PreventionSmith+Nephew
SecuraDownload Download Download 


Terumo
SurTractTerumo
SurGuard3 NeedleTerumo
Surshield-PURDownload Download Download 

Be social & follow: Copyright © 2025, All rights reserved.
NDC, Inc.
402 BNA Drive, Ste. 500
Nashville, TN 37217


unsubscribe from all emails update subscription preferences Communication Type:
Product Flyer
V#10030
-
10/9/2025
Title: Aspen Surgical V#10234
Details:
Concordance Team,
Please see below and attached from Aspen Surgical V#10234 about a price increase:
Effective January 1, 2026, price increases will affect products throughout the Aspen Surgical
portfolio. We have enclosed an updated price list.Our decision to adjust pricing is not taken lightly. The following factors have contributed to the
increase:
• Increased Costs: The costs of transportation, raw materials, sterilization, and labor have
all increased significantly in recent years.
• Inflation: Broader economic inflation has impacted our cost structures. We have
absorbed most of these costs but cannot absorb all the cost increases we have
experienced.
• Mitigated Past Price Increases: In 2026, we selectively raised prices on a few
product codes to minimize customer impact.We understand the impact of price adjustments on your operations and have implemented several
strategies to mitigate these effects, including:• Insourcing Products: To improve cost control measures.
• Product and Packaging Redesign: To optimize efficiency.
• Evaluation of Raw Material Suppliers: To ensure cost-effectiveness without
compromising quality.
• Investments in eCommerce Capabilities: To streamline purchasing processes.Please update your pricing system to reflect the attached pricing for all orders placed after
January 1, 2026. This will help prevent any discrepancies and ensure a smooth transition.Communication Type:
pricing_changes
V#10234
-
10/8/2025
Title: Cardinal V#10801
Details:
Concordance Team,
Please see below and attached from Cardinal V#10801 about their upcoming holiday schedule. Please contact Mary Reinhart if you have any questions.
Thanksgiving Holiday Closure
Due to the upcoming holiday 11/27/2025, your Cardinal Health replenishment center will be closed.
- For the holiday week of the 24th, please place your Monday and Tuesday orders as normally scheduled.
- Please place the standard orders, that you would place on the 26th – 28th, by Wednesday, November 19th , to supplement orders that would have been placed on the 26th- 28th.
Orders placed after your normal cut-off time will be processed on Monday, December 1st.
For standing delivery appointments (SDAs), adjustments will be coordinated by your transportation replenishment center leader.
If you have further questions, please contact Customer Service at 800.635.6021. Thank you for your continued business and your cooperation during this holiday time.
Communication Type:
Holiday Schedule
V#10801
-
10/7/2025
Title: Tidi V#10260
Details:
Concordance Team,
Please see below and attached from TIDI V#10260 regarding 16 Novaplus SKU's that will be discontinued. I have attached a usage file for the past six months. Please reach out to Heather Burkett if you have any questions. Thanks!Please see the attached notice regarding an upcoming change to a Vizient contract. Note: each item being discontinue has a direct cross reference in our Posey brand and although inventory of NovaPlus has not fully depleted, it will be used to fill orders currently in our system. Also, end-user customers are currently being made aware of the change, as well (9.22 letter).
We appreciate your help with the transition and please let us know any questions or concerns.
Thank you,
Denise Sitzberger
National Account ManagerCell: 616.450.5614
Communication Type:
Discontinuation Notice
V#10260
-
10/7/2025
Title: Welch Allyn V#10834
Details:
Concordance Team,Please see the attached notification on the End of Manufacturing notice for the Welch Allyn ProBP3400 device. The letter contains the specific dates as well as the replacement options moving forward. I have attached a usage file for the last 6 months for the items listed. Please reach out to Tricia Wentling if you have questions. Thanks!
Communication Type:
Discontinuation Notice
V#10834
-
10/6/2025
Title: HR Healthcare V#11338
Details:
Concordance Team,
The items below have been discontinued from HR Healthcare V#11338. Per the attached report, there have been no sales in the past six months for any of these items.
S#400454 Mfg #90104 – discontinued; no replacement
S#400456 Mfg #90208 – discontinued; no replacement
S#400458 Mfg #90316 – discontinued; no replacement
S#400459 Mfg #90432 – discontinued; no replacement
S#400462 Mfg #90515 – discontinued; no replacement
S#400464 Mfg #90604 – discontinued; no replacement
Communication Type:
Discontinuation Notice
V#11338
-
10/6/2025
Title: BD V#10125
Details:
Concordance Team,
Please see the attached customer notice and customer listing from BD V#10125. There is a national shortage of .2-micron filters with no recovery date (See attached customer letter). The highlighted items below are being allocated:10010454
134789
24340007
403201
c24101e
n/a
11532269
177978
already allocated
10015012
231831
already allocated
243010007t
231646
24320007
244946
already allocated
20350e
733279
already allocated
20027e
218795
already allocated
42933e
217016
20350et
318957
10013902
223449
Please contact Heather Burkett if you have further questions.
Communication Type:
Allocation Notification/Update
V#10125
-
10/6/2025
Title: Greiner Bio-One V#11403
Details:
Concordance Team,
See below from Greiner Bio-One V#11403 regarding a product update to C# 220472 and 359455.
Please see the attached notification from the Griener Bio-One Product Management team regarding a change to the VACUETTE® Blood Culture Holder item # 450181.
Beginning in October of this year, we are adding a prominent single-use symbol and replacing the VACUETTE® logo with a Greiner Bio-One logo. Additionally, there will be a minor change to the thread.
Important Note: These changes will not affect the product's performance, form, fit, or function, and are being made to better align with other holder products and therefore optimize compatibility across our line.
Please review the attached document for more detailed information. If you have any questions or concerns, do not hesitate to reach out.
Please contact Heather Burkett if you have further questions.
Communication Type:
Product Changes
V#11403
-
10/3/2025
Title: Medline V#10605
Details:
Concordance Team,
Please see below from Medline V#10605. C#805747 is being discontinued. A usage report for the past six months is attached.
Hello Distributor partners,
ISO1003 item is going through the Item Conversion process.
Original Item: ISO1003- ISOLATION BAG 18X18.5
New item: DYNJSD1003- BAG, ISOLATION,20X20, INVISISHIELD
This item will runout at the end of this month.
Please contact Heather Burkett if you have further questions. Thanks!
Communication Type:
Discontinuation Notice
V#10605
-
10/3/2025
Title: Abbott Point of Care V#12125
Details:
Concordance Team,
CHS#390271 #03P93-25 from Abbott Point of Care V#12175 has been discontinued with no replacement. CHS#390271 has been placed in item inactivation. There have been no sales of this item in 2025.
01-2024-10 US Customer Letter CK-MB BNP hCG 19 Jan 2025
01-2024-09 US Customer Letter troponin 19 Jan 2025
Please contact Mary Reinhart with questions. Thanks!
Communication Type:
Discontinuation Notice
V#12125
-
10/3/2025
Title: B.Braun V#10508
Details:
October Allocation Files
Please see allocation file from B Braun, V#10508 for October. Please reach out to Heather Burkett if you have any questions.
Communication Type:
Allocation Notification/Update
V#10508
-
10/2/2025
Title: Nestle V#10183
Details:
Concordance Team,
Please see the attached email and update below from Nestle V#10183 regarding a product update. If you have questions, please contact Mary Reinhart.
Novasource® Renal Vanilla Flavor Update
In order to ensure all ingredients in our products align to the same standards,
consumer preferences, and provide the consistent supply needed to support the
demand, we have updated our vanilla flavor in Novasource® Renal
Vanilla. Consumers may notice a slight difference in flavor, more aligned to other
vanilla products in our portfolio. There are NO changes to the nutrition
information, label, packaging or codes.Novasource® Renal with the new vanilla flavor will start flowing into the market in
November of 2025, depending on Nestle’s inventory. These dates are subject to
change.
Novasource® Renal Mocha and Strawberry remain unchanged
For questions regarding these changes, you may contact Nestlé Health Science Distributor Manager
directly or call our consumer hotline at 1-800-422-2752. Thank you for your continued business, and we
look forward to our ongoing partnership!Communication Type:
Product Changes
V#10183
-
10/2/2025
Title: Abbott Nutrition V#10575
Details:
Details:
Attached you will find the standard weekly inventory update letter you have been receiving:
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Communication Type:
Product Inventory Update
V#10575
-
10/1/2025
Title: Baxter V#10797
Details:
Concordance Team,
Baxter is discontinuing some IV products that had been imported on a temporary basis earlier this year in response to last year’s Hurricane Helene. The entire list is in the attached PDF document.
SALES: Of the item codes set up in VAI, the attached usage file shows just two customers that placed an order over the past six months.
Per the letter, “These product codes that were authorized for temporary importation and distribution by Baxter are listed in Table 1. Effective October 31, 2025, inventory of these temporary importation codes will be depleted and no longer available for sale. Please update your ordering systems to the Baxter U.S. Product Codes, which begin with “2B”. as listed in Table 2.
Communication Type:
Discontinuation Notice
V#10797
-
09/30/2025
Title: Procter and Gamble V#10097
Details:
Hello Concordance Team,Product # 03498 (C#351912) from P&G V#10097 is no longer available for hospital use and has been discontinued for use in Acute Care. The usage file is attached.Please contact Tricia Wentling if you have any questions. Thanks!Communication Type:
Discontinuation Notice
V#10097
-
09/30/2025
Title: Mozarc Medical V#13599
Details:
Concordance Team,
Please see the IMPORTANT UPDATE below and updated discontinuation letter from Mozarc Medical about an EARLIER DISCONTINUATION DATE on the Legacy Mahurkar line of catheters
An updated usage report is attached listing customers who are still purchasing the Legacy line.
- PURCHASING: Please work with Nathan Orange to find out which purchase orders will need canceled per his reference to roughly $11K worth of orders on Legacy products that they will not be able to fill.
- SALES/CUSTOMER RELATIONS: Please work with your customers on the attached usage report to get them converted to the new Elite items and cancel open orders of Legacy products that cannot be filled.
- ITEM MAINTENANCE: For Legacy items not yet discontinued in the system and are not on any open orders, please discontinue them. For Legacy items currently on open orders, please discontinue them once open orders have been canceled.
Mahurkar Legacy to Elite comparison
Mozarc V#13500 disco item list cross to CHS#
Communication Type:
Discontinuation Notice
V#13599
-
09/29/2025
Title: Nestle V#10183
Details:
Here’s the Nestle Inventory Letter for this week. Please contact Mary Reinhart if you have any questions.
1. Beneprotein is still on the report
2. Boost Chocolate is still on the report
3. Compleat Pediatric Peptide 1.5 250 ml is still on the report
4. Thickenup Clear 4.4 oz canister is still on the report
5. Impact Peptide 250 and 1000 ml are still on the report
6. DIABETISOURCE® AC, SpikeRight® PLUS 4 x 1500 mL UltraPak® bags was added
7. COMPLEAT® ORIGINAL 1.0, Spike Right® PLUS 6 x 1000 mL UltraPak® bags was added
8. COMPLEAT® PEDIATRIC ORIGINAL 1.5 Fruit Medley 24 x 250 mL carton was added
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Communication Type:
Product Inventory Update
V#10183
-
09/26/2025
Title: Midmark Corporation V#10987
Details:
Concordance Team,
Please see below from Midmark Corporation V#10987. Please contact Mary Reinhart with further questions.
See attached a communication regarding a change to Midmark’s pricing strategy for our custom casework product offering. Following your review of this letter let me know if you have any questions. If needed, I’ll be happy to discuss live with you on a call.
Communication Type:
pricing_changes
V#10987
-
09/26/2025
Title: Roche Diagnostics V#10603
Details:
Hello,
Please see the email and attachments below from Roche V#10603. The following items are being discontinued:

Please contact Tricia Wentling with further questions.
We are planning to discontinue the Accutrend meter at the end of this year. I just learned that our Marketing team proactively sent the attached Customer letter to current Accutrend customers at the end of August, but also sent a Distributor letter to all of the Distribution SAP sold-to addresses we have on file, which are your DC's. (Unfortunately, Roche Regulatory requires mailings to the SAP sold-to addresses.) The problem is that our Marketing team did not provide these letters to the National Account Managers in advance so we could get them to you/HQ's prior to them being received by your DCs or the Customer letters being received by end customers! Apologies if this has created any confusion!
Also, as clarity, only the Accutrend METER is being discontinued at the end of this year. Unfortunately, the letters state that the meter will be phased out as of December 2025, but goes on to say we should have 6-months of inventory as of September....My recommendation would be for you to set the meter for discontinuation effective 1/1/26. Any additional meters we have leftover past December will be used for technical issue/warranty replacements. The Distributor letter also asks that you send the Customer letter out to Accutrend customers, but since Roche has already mailed the Customer letter to every address where we've drop-shipped an Accutrend meter, that's likely not necessary, and you could use the Customer letter with customers who might have questions and for your field team's reference.
Unfortunately, when the Accutrend meter is discontinued, Roche really doesn't have a comparable product to replace it. However, the strips and controls will continue to be available to support existing customers, as they are not slated to be discontinued until 2027.
Again, apologies that these customer letters were mailed before you had a chance to receive them. Please let me know if you have any questions or if there is anyone else at Concordance that I should send this information to.
Thanks!
Shelley Miller
National Account Manager, Point of Care
Roche Diagnostics Corporation
9115 Hague Road
Indianapolis, IN 46250-0457
Phone: 317.504.8164
Email: shelley.miller@roche.com
Communication Type:
Discontinuation Notice
V#10603
-
09/25/2025
Title: Abbott Nutrition V#10575
Details:
Attached you will find the standard weekly inventory update letter you have been receiving:
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Communication Type:
Product Inventory Update
V#10575
-
09/25/2025
Title: Kenvue V#10860
Details:
Concordance Team,
Please see the attached for Kenvue’s New Sun Care & Tylenol Products launching Q4 2025. All pricing and product information is included.
Please contact Tricia Wentling with any further questions.
Communication Type:
New Product Launch
V#10860
-
09/25/2025
Title: Sekisui V#10161
Details:
Please see the below and attached end user promo from Seisui V#10161.
We are excited to announce an OSOM® Combo Special Promotion will be starting October 1st! Please see the details below.
End User Promo
- Qualifying products include:
- OSOM® Flu SARS-CoV-2 Combo Test (cat. no. 1080)
- We are offering get 7 kits for the price of 4 to end-users.
- The promotion will run October 1st – December 31st, 2025.
- This is fulfilled by SEKISUI Diagnostics so there is nothing to do on your end except spread the word to your customers. Flyer is attached for dissemination to both your reps and end users.
Please contact Heather Burkett if you have any questions.
Communication Type:
End User Promo
V#10161
-
09/24/2025
Title: Abbott Point of Care V#12125
Details:
Please see the email below from Abbott Point of Care V#12125 about their i-STAT software update. Contact Mary Reinhart if you have further questions.
New i-STAT System software will be available the week of:
- October 14, 2025 for i-STAT 1
- October 27, 2025 for i-STAT Alinity
Prepare now for new i-STAT software
Register to ensure access to software. If you're not responsible for i-STAT System devices, please forward this email to the appropriate colleague.
Bookmark the software page and log in on this page to access your software after the release date.
No longer an i-STAT customer? Change your profile and communication preferences here.Thank you for choosing the i-STAT System as your point-of-care solution.- Current i-STAT 1 software will expire December 10, 2025.
- Current i-STAT Alinity software will expire January 14, 2026.
For in vitro diagnostic use only.Communication Type:
Product Update
V#12125
-
09/24/2025
Title: Detecto V#10793
Details:
Please see the details on new products below from Detecto V#10793.I just wanted to make sure you had the details on the 3 new Detecto models that are now released into the market. We have two new Apex scale models (featuring our new handrails), models ApexH-AC and ApexH-SH-AC. This means that we now offer a handrail on both the mechanical and sonar version scale models of the Apex!We have also officially released the new pediatric medical cart – model PSCART. This great new pediatric cart offers two locking wheels and one steering lock for maneuverability and safety, along with two shelves for storage. I have enclosed the brochure for details on specifications and features.
Please reach out to Mary Reinhart or Terry Sims if you have any questions.
Terry Sims
Regional Sales Manager - East
tsims@detecto.com
Communication Type:
New Product Launch
V#10793
-
09/23/2025
Title: Medtronic V#10255
Details:
Vendor Item List Sales History with Units - 2025-09-22T094910.582
Concordance Team,
Please see the email below from Medtronic:
Dear Distributor Partners,
Our safety stock and supply have recovered on the TruClear™ Elite Soft Tissue Mini shaver, product code 72202536.
As such, we have removed the “Drop Ship Only” classification on this product code.
Distributors may now purchase and stock product code 72202536, effective immediately.
Product Code: 72202536
Description: TruClear™ soft tissue shaver mini
Sell UOM: Each (1 Each)
Medtronic Division: Med Surg / Surgical / GYN
Please forward this message to the appropriate materials management and product master departments within your organization.
Please contact Customer Service at rs.covidiencustomerservice@medtronic.com or (800) 962-9888 with any order related questions.
Thank you.
Brian Hannan
Director, Distributor Sales Operations
Medtronic
60 Middletown Ave. | North Haven, CT 06473 | U.S.A.
Mobile: 203-451-1971
E-Mail: Brian.H.Hannan@Medtronic.com
Communication Type:
Product Inventory Update
V#10255
-
09/23/2025
Title: Stryker V#10912
Details:
Please see the attached recall notification regarding the expansion of the SafeAir recall that was initiated October 2024.
Included is the Customer Notification Letter – SafeAir Expansion 1.pdf – please forward this customer-facing communication to all affected mutual end-customers.
This has been shared with the Concordance Recall Coordinator who will properly handle this with all necessary customers and internal parties.
Communication Type:
Recall Notification
V#10912
-
09/23/2025
Title: TIDI V#10260
Details:
Please see below regarding a discontinuation notice from TIDI, V#10260. Below are the C#’s discontinued with possible subs for each. I have also included a file with usage for the past 6 months. Please contact Heather Burkett if you have any questions. Thanks.
S#960666 (Mfg #4644) discontinued – no replacement
S#663765 (Mfg #4645) discontinued – no replacement
S#620351 (Mfg #4646) discontinued – no replacement
S#168841 (Mfg #6218) discontinued – replaced by S#254805 (Mfg #6145)
S#183063 (Mfg #6218L) discontinued – replaced by S#254805 (Mfg #6145)
S#120382 (Mfg #2797) discontinued – replaced by S#883439 (Mfg #2755)
Communication Type:
Discontinuation Notice
V#10260
-
09/23/2025
Title: Hemocue V#10452
Details:
Please see below from Hemocue, V#10452. These items are all listed as dropship only.
Please note that the following (all) StatSpin centrifuges will have a six (6) month warranty starting on PO’s received after September 30th, 2025. Please contact Heather Burkett with any questions.
C#
ITEM NUMBER
DESCRIPTION
261308
SSX2
StatSpin Express 2
203161
SSX3
StatSpin Express 3
226160
SSH4
StatSpin Express 4
323138
SSMP
StatSpin MP Multipurpose Centrifuge
281926
CF02
StatSpin CytoFuge® 2
Communication Type:
Product Update
V#10452
-
09/22/2025
Title: Kimberly Clark V#10765
Details:
Concordance Team,
See attached/below for a list of consumer products and brands now available from Kimberly Clark V#10765. For SAMPLES, reach out to Tawni Ruffino at Tawni.Ruffino@kcc.com.

Communication Type:
New Product Launch
V#10765
-
09/22/2025
Title: Nestle V#10183
Details:
Here’s the Nestle Inventory Letter for this week.
1. Beneprotein is still on the report
2. Boost Chocolate is still on the report
3. Compleat Pediatric Peptide 1.5 250 ml is still on the report
4. Thickenup Clear 4.4 oz canister is still on the report
5. Impact Peptide 250 and 1000 ml are still on the report
6. DIABETISOURCE® AC, SpikeRight® PLUS 4 x 1500 mL UltraPak® bags was added
7. COMPLEAT® ORIGINAL 1.0, Spike Right® PLUS 6 x 1000 mL UltraPak® bags was added
8. COMPLEAT® PEDIATRIC ORIGINAL 1.5 Fruit Medley 24 x 250 mL carton was added
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Communication Type:
Product Inventory Update
V#10183
-
09/19/2025
Title: Abbott Nutrition V#10575
Details:
Attached you will find the standard weekly inventory update letter you have been receiving:
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Communication Type:
Product Inventory Update
V#10575
-
09/19/2025
Title: Nestle V#10183
Details:
Attached is the Nestle inventory letter for this week.
1. Benecalorie is still on the report
2. Beneprotein is still on the report
3. Boost Chocolate is still on the report
4. Compleat Pediatric Peptide 1.5 250 ml is still on the report
5. Thickenup Clear 4.4 oz canister is still on the report
6. Impact Peptide 250 and 1000 ml are still on the report
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Communication Type:
Product Inventory Update
V#10183
-
09/19/2025
Title: Midmark V#10987
Details:
Hello.
Please see attached notification of product discontinuations.
Thank you.
Designing better care.®
Terry Campbell
Corporate Accounts Specialist
Midmark Corporate Accounts Department

T: 1.800.MIDMARK
D: 937.526.8224
midmark.com
Communication Type:
Discontinuation Notice
V#10987
-
09/12/2025
Title: Coloplast V#10581
Details:
Freedom Estimated Runout Excel 05.06.2025
PM-35881 Freedom Clear discontinuation conversion list_HCP_F
PM-35885 Freedom Clear discontinuation Conveen Optima HCP fact sheet_F
USCC_Freedom Clear Discontinuation_Letter_PM-38147_F (Excel)
USCC_Freedom Clear Discontinuation_Letter_PM-38147_F (PDF)
Concordance Team,
Coloplast V#10581 is discontinuing a line of male catheters now and into 2026.

Item Maintenance has discontinued the following items in our system so far and will address the rest of the items in December and in 2026 in accordance to the runout schedule provided by Coloplast. There has bee NO usage in the past six months.

Communication Type:
Discontinuation Notice
V#10581
-
09/12/2025
Title: Medtronic V#10255
Details:
Vendor Item List Sales History with Units 3.12.25-9.11.25
Medtronic Med Surg Shiley Product Notification May2025
Medtronic Med Surg Shiley Product Notificaton May25
Concordance Team,
Medtronic V#10255 has discontinued items listed below and Item Maintenance has placed them in inactivation accordingly. As noted below, the vendor did not provide any direct replacements. A usage file with customer detail is attached for your use.
S#722009 #86045 disc no replacement
S#721407 #86048 disc no replacement
S#721621 #86050 disc no replacement
S#984351 #86056 disc no replacement
S#722041 #86057 disc no replacement
S#816942 #86003 disc no replacement
Communication Type:
Discontinuation Notice
V#10255
-
09/12/2025
Title: J&J V#10196
Details:
US HN Customer Letter_FINAL_042025
Vendor Item List Sales History with Units - 3.10.25-9.10.25
Concordance Team,
Item Maintenance has discontinued the below list of Mfg Item Numbers per the attached communication from Johnson & Johnson V#10196. This list also shows the suggested J&J alternative items numbers (crossed to Concordance item numbers).
Only the items in bold below had usage over the past six months. See the attached usage report with customer detail for your reference.

Communication Type:
Discontinuation Notice
V#10196
-
09/12/2025
Title: J&J V#10196
Details:
Customer Letter_JJMT Biosurgery UoM WAVE2_SEP 2025_FINAL
Vendor Item List Sales History with Units - 03.10.25-09.9.25
Concordance Team,
Please see the unit of measure (UOM) changes described in the below J&J V#10196 email.
The mfg items have been crossed to Concordance item numbers below. Please refer to the attached usage report for a customer listing.

Communication Type:
Product Changes
V#10196
Communication Type:
Product Update
V#10196
-
09/12/2025
Title: Kate Farms V#12409
Details:
Please see the attached memo that provides information about upcoming changes to ordering Functional Formularies and Real Food Blend. Please share this information with the right teams internally to begin planning.
Let me know if you have any questions or need more information. We will continue to have open communications and answer any and all questions that come up.
Please contact Mary Reinhart with any questions.
Communication Type:
Product Changes
V#12409
-
09/12/2025
Title: Essity V#10272
Details:
Please see below from Essity, V#10272 regarding items that could possibly be used as a sub while Dermarite items are being recalled- cross reference attached.
We were provided feedback from the field that DermaRite is going through a Recall (link below). If you encounter supply issues and need to find a substitute offering, please consider Essity products to help support customer needs. I have attached a cross reference file for your review.
Please see link on the Dermarite recall- https://dermarite.com/category/press-release/. I have attached a listing with items affected and customers who have purchased in the past 6 months.
Let me know if you have any questions or issues. Reach out to Heather Burkett if you have any questions. Thanks!
Communication Type:
Recall Notification
V#10272
Communication Type:
Substitution Option
V#10272
-
09/12/2025
Title: Pfizer V#12114
Details:
Per the information below, the following items are discontinued and have been placed in item inactivation.
S#006477 #00409-2267-20 disc no replacement
S#282943 #60793-0916-03 disc no replacement
No Longer Available for Shipping
July 14, 2025
Dear Valued Customer,
Please click the link below to review important information regarding Products no longer available for shipping from Pfizer Hospital.
Click here to view the Customer Letter
Please contact Customer Service at 1-844-646-4398 should you have any questions.
Sincerely,
Pfizer HospitalCommunication Type:
Discontinuation Notice
V#12114
-
09/12/2025
Title: Shamrock Scientific V#11111
Details:

Shamrock Labels is proud to serve Vizient members with:
- Labels for every department
- Wristbands for patient identification
- Smart printers and RFID support
- Streamlined ordering with Vizient contract pricing
- Additional benefits available through Novaplus
Communication Type:
New Product Launch
V#11111
-
09/12/2025
Title: Nestle V#10183 - NHSc Weekly Inventory Update
Details:
Attached is the Nestle inventory letter for this week
1. Benecalorie is still on the report
2. Beneprotein is still on the report
3. Boost Chocolate is still on the report
4. Compleat Pediatric Peptide 1.5 250 ml is still on the report
5. Thickenup Clear 4.4 oz canister is still on the report
6. Impact Peptide 250 and 1000 ml are still on the report
Please contact Mary Reinhart if you have any questions.
Communication Type:
Product Inventory Update
V#10183
-
09/12/2025
Title: Detecto V#10793
Details:
Please see below documents from Detecto, V#10793 if needed.
DETECTO Clinical Department Powerpoint
Scaletronix Discontinued Items- Crossed to Detecto
If you have any questions reach out to the below contact. Thanks,

Communication Type:
Discontinuation Notice
V#10793
Communication Type:
Product Changes
V#10793
-
09/11/2025
Title: BSN Medical V#10231
Details: Please see link for new product offering from BSN Medical, V#10231. Please reach out to Item Maintenance for set up. If you have any questions please reach out to Heather Burkett.
Communication Type:
New Product Launch
V#10231
-
09/11/2025
Title: BD V#10128
Details:
Please see below, Veritor Promotion from BD Diagnostics. If you have any questions, please reach out to Heather Burkett.


Communication Type:
End User Promo
V#10128
-
09/11/2025
Title: Invacare Continuing Care V#10748
Details:
Holiday Schedule for Sept/Oct 2025

Invacare and subsidiary offices will be CLOSED on
SEPTEMBER
Tuesday, September 23rd
Wednesday, September 24th

Communication Type:
Holiday Schedule
V#10748
-
09/11/2025
Title: BD V#10125
Details:
Good afternoon!
Please see attached allocation updates from BD, V#10125. Item listed below have been removed. Attached is an updated customer listing.
Please reach out to Heather Burkett you have any questions. Thanks,
309657
173729
3ML LUER LOK SYRINGE
Remove from allocation
367365
239308
WINGSET PBBCS UTW PP 21X.75 12 LUER
Remove from allocation
367324
116599
Wingset PBBCS 23x.75 12 w/o Luer
Remove from allocation
930415
317166
3mL ORANGE STERILE SOLUTION/APPLICATOR
Remove from allocation
Communication Type:
Allocation Notification/Update
V#10125
-
09/11/2025
Title: Phase Scientific V#12840
Details:
Please see below for new product offering from Phase Scientific. This is a new one of a kind product, never been on the market before.
Please see link for Sales sheet here.
Please see link for CLIA waiver here.

Communication Type:
New Product Launch
V#12840
-
09/5/2025
Title: Abbott V#10575
Details:
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File 9.4.25 Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Communication Type:
Product Inventory Update
V#10575
-
09/5/2025
Title: Nestle V#10183
Details:
Attached is the Nestle Inventory Letter for this week.
- Benecalorie is on the report
- Beneprotein packets is still on the report
- Boost Chocolate was added to the report
- Boost Vanilla is on the report
- Compleat Pediatric Peptide 1.5 250 ml is still on the report
- Boost GC Max 30 gm Protein Vanilla is still on the report
- Boost GC Max 30 gm Protein Chocolate is still on the report
- Diabetisource AC 1500 ml was added to the report
- Thickenup Clear 4.4 oz canister was added to the report
- Impact Peptide 250 and 1000 ml are still on the report
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Experience our Support Site
Communication Type:
Product Inventory Update
V#10183
-
09/5/2025
Title: Belimed V#13593
Details:
Steelco/Belimed
we have finally reached the date for the merger and integration of Belimed Inc and Steelco-USA Inc. Going forward we will be operating as SteelcoBelimed Inc, and attached is a letter from our Co-CEO's announcing this change.
We are still in the process of getting copies of our W-9 with the new name, but our tax ID will remain the same. As we have these documents to share I will continue to reach back out in the coming weeks.
We look forward to continuing our relationship with Concordance as we look to strengthen and grow our position in the US market as SteelcoBelimed.
Regards,
Chris
Chris Ginnett
Director, Supply Chain & Logistics
Belimed Inc
8351 Palmetto Commerce Parkway • Ladson, SC 29456 • USAT +1 843 216 7424 • M +1 843 830 0877
chris.ginnett@belimed.com
Communication Type:
Updated Supplier Information
V#13593
-
09/5/2025
Title: MicrocareV#10201
Details:
Hi Mary,
Below is a link to the 2-Minute Game Plan video we launched via Repertoire for our Spec Clean Pre-Treatment Gel.
Please feel free to share with your sales team.
Charlie Campbell
Key Account Manager
6120 East 58th Avenue
,
Commerce City
,
CO
,
80022
From: Dail-eNews <dailenews@sharemovingmedia.com>
Sent: Tuesday, September 2, 2025 1:31 PM
To: Charlie Campbell <CharlieCampbell@microcare.com>
Subject: New 2-Minute Game Plan – MicroCare Medical’s Spec Clean™ Pre-Treatment Gel SprayDail-eNews for September 2, 2025
Click here to view this message in browser
Tuesday, September 2, 2025
New 2-Minute Game Plan – MicroCare Medical’s Spec Clean™ Pre-Treatment Gel Spray
A new 2-Minute Game Plan video is available from MicroCare Medical on their Spec Clean™ Pre-Treatment Gel Spray.
Get the new 2-Minute Game Plan now!
Did you know? You can access this video as well as previous Game Plan videos via the RepConnect app for iOS mobile devices. To download the app on your iOS mobile device, search for “Repconnect” (one word) in the App Store, or click here.
InspiroGene by McKesson launches InspiroCare™ Patient Hub for Cell and Gene Therapy
InspiroGene™ by McKesson, a dedicated business focused on supporting the commercialization of cell and gene therapies (CGTs), launched InspiroCare™, a purpose-built patient hub designed to simplify the complex journey of cell and gene therapies. Leveraging the proven expertise of Biologics by McKesson, InspiroCare brings together decades of patient support know-how with a deep understanding of the unique CGT patient journey to help ensure patients, caregivers and providers get the support they need...
BD expands peripheral artery disease awareness efforts with ‘Love Your Limbs’ community screening initiative
In recognition of Peripheral Artery Disease (PAD) Awareness Month, BD is shining a spotlight on a serious yet often overlooked circulatory disorder that restricts blood flow to the limbs and is a leading cause of preventable amputations. As part of the BD commitment to PAD awareness, the company launched the Love Your Limbs™ PAD Community Screening Initiative to expand access to PAD screening by partnering with physicians to offer free ankle-brachial index (ABI) screenings, which are essential, non-invasive test that can detect PAD in less than 15 minutes...
Thermo Fisher Scientific completes acquisition of Sanofi’s Ridgefield, New Jersey Site
Thermo Fisher Scientific Inc. announced the completion of its acquisition of Sanofi’s state-of-the-art sterile fill-finish and packaging site in Ridgefield, New Jersey, marking an expansion of the companies’ strategic partnership to enable additional U.S. drug product manufacturing. The terms of the deal were not disclosed. The Ridgefield facility is now part of Thermo Fisher’s pharma services business within its Laboratory Products and Biopharma Services segment...
Federal appeals court rules against Trump’s ‘reciprocal tariffs’
A divided U.S. appeals court ruled on Friday that most of Donald Trump’s tariffs are illegal, according to Reuters. The court allowed the tariffs to remain in place through October 14 to give the Trump administration a chance to file an appeal with the U.S. Supreme Court. Last Friday’s 7-4 appeals court decision tossed out a part of that ruling striking down the tariffs immediately, however the court’s ruling doesn’t cover all of President Trump’s tariffs...
Prostate cancer incidence climbs, mortality declines slow
The American Cancer Society (ACS) released Prostate Cancer Statistics, 2025, a report on current prostate cancer occurrence and outcomes in the United States. According to the study, prostate cancer incidence rates have reversed from a decline of 6.4% per year during 2007 through 2014 to an increase of 3.0% annually during 2014 through 2021, with the steepest increase (4.6%-4.8% per year) for advanced-stage diagnoses...
Connect with your Customers!
Interested in advertising in one of our publications, submitting content, or working with us to create engaging content that features your business?
Aili Casey
Director of Business
Development -
Repertoire
Anna Miller
Director of Business
Development -
Journal of Healthcare
Contracting
Subscribe to Dail-eNews to receive the latest news in the healthcare supply chain!
Copyright 2025 Share Moving Media
Communication Type:
New Product Launch
V#10201
-
09/5/2025
Title: Nestle V#10183
Details:
Attached to this email, you will find the 9/1 communication and the related documents. Spec sheet I understand this might have caused some inconvenience, and I am truly sorry for any confusion this may have caused.
If you have any questions or need further assistance with this information, please don't hesitate to reach out. I'm here to help in any way I can.
Thank you for your understanding and patience.
Amy Sauber
Amy Sauber, RDN, LDN
Manager, Sales Support Team
Nestlé Health Science U.S.
CONFIDENTIALITY NOTIFICATION: This email message and any attached documents are intended only for the use of the intended recipient(s) and may contain information that is confidential. If you have received this email in error, please notify the above sender and delete it from your computer.
Communication Type:
New Product Launch
V#10183
-
09/4/2025
Title: Midmark V#12345 - Pricebook Updates
Details:
Concordance Team,
Midmark has notified us of a Price Book Revision for September2025 for Midmark Product +. Please see the link below for further information.
Password: MidDlrPricing
With further questions, please reach out to Mary Reinhart.
Kind Regards,
Jamie Miller
Communication Type:
pricing_changes
V#10987
-
09/3/2025
Title: J&J V#10196
Details:
US HN Customer Letter_ Final_042025_v2
JJMT - Product Discontinuation Notice - April 2025_V2__with CHS item numbers
Vendor Item List Sales History with Units - 3.3.25-9.2.25
Concordance Team,
J&J V#10196 announced the discontinuation of several surgical products with various effective dates. Refer to the attached PDF letter for complete details.
- See attached PDF letter for an item list and some suggested replacements.
- See attached J&J Excel file with Production Discontinuation item listing—Concordance item numbers have been added.
- See attached 6-month usage report for items and customer listing.
- Women’s health and hernia mesh offerings – ARTISYN™ Y-Shaped Mesh, GYNECARE GYNEMESH™ PS, and ETHICON PHYSIOMESH™ discontinued by December 2025.
- MEGADYNE™– discontinuation of MEGADYNE™ MEGA SOFT™ Universal and Universal Plus Reusable Patient Return Electrodes by December 2025 as well as discontinuation of MEGADYNE ™ MEGA SOFT ™ Cables and Accessories and a select group of MEGADYNE ™ products.
- Select endomechanical offerings - J&J will be discontinuing specific models of their older generation surgical staplers, as well as several trocars, endo sutures, and other ligation products and accessories by September 2025 (select SKUs) or September 2026 (select SKUs).
Communication Type:
Discontinuation Notice
V#10196
-
08/29/2025
Title: Abbott V#10575
Details:
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File 8.28.25 Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Chad Truini
Sr. Business Analyst
Commercial Operations
Abbott Nutrition
3300 Stelzer Road
Columbus, OH 43219
O:
+1 614-624-0239
Communication Type:
Product Inventory Update
v#10575
-
08/29/2025
Title: Nestle V#10183
Details:
I hope this message finds you well. Attached, you will find a communication about Beneprotein that contains some important information. If you have any questions or need further clarification, please do not hesitate to contact our product information line at 800-422-ASK2. Alternatively, you can reach out to your Distributor Manager who will be more than happy to assist you.
Thank you for your attention to this matter.
Best regards,
Amy Sauber
Amy Sauber, RDN, LDN
Manager, Sales Support Team
Nestlé Health Science U.S.
CONFIDENTIALITY NOTIFICATION: This email message and any attached documents are intended only for the use of the intended recipient(s) and may contain information that is confidential. If you have received this email in error, please notify the above sender and delete it from your computer.
Communication Type:
Product Update
V#10183
-
08/29/2025
Title: GOJO V#10354
Details:
GOJO UPDATE - 515mL Bottle Bracket Portfolio Evolution
5704-06 PIC
Concordance Team,
GOJO has referenced two discontinued items on the attached PDF notification, but only 5704-06-BLU (C#202550) is set up in our system. There were no customer sales in the past six months. for this item.
The other portion of GOJO’s attached notification refers to two new SKUs. If customers request to purchase them, contact Item Maintenance to set them up in our system.
Communication Type:
Discontinuation Notice
V#10354
Communication Type:
New Product Launch
V#10354
-
08/28/2025
Title: Ecolab V#10929
Details:
August Notice Ecolab Product Discontinuation effective 11.2025
Concordance Team,
With regards to the Hand Hygiene portfolio, Ecolab V#10929 is discontinuing four SKUs effective 11/1/25 per the attached notification. Two of the four are set up in our system, one of which had one customer sale over the past six months.

Communication Type:
Discontinuation Notice
V#10929
-
08/27/2025
Title: SC Johnson V#12219
Details:
Vendor Item List Sales History with Units - 2.26.25-8.26.25
Concordance Team,
Per the attached letter “08.2025_Bactoshield Update”, SC Johnson is transitioning one of their trusted brands, Bactoshield®, to a new formulation beginning with our production runs in September 2025.
What You Can Expect:
- Color Change: The product will shift from a pale pink to an amber hue. We can confirm that no dyes have been added to the new formulas.
- Fragrance Update: For consistency across our offerings, both the 2% and 4% formulas will now include a light fragrance.
- Ingredient Changes: Please review and update your records with the new Safety Data Sheets (SDS). (qty 2-SEE ATTACHED)
You can expect to begin receiving the new formulation with orders fulfilled from September 2025 onward.
SC Johnson provided a list of affected item numbers in the below email, and CHS numbers have been crossed:

ATTACHMENTS:
- Bactoshield Update notification
- SDS sheets (qty 2)
- Six-month customer usage report
Communication Type:
Product Changes
V#12219
-
08/26/2025
Title: Medtronic V#10255
Details:
Concordance Team,
Upon notification from Medtronic V#10255, the allocation has been removed in our system for the following two items. A customer listing is attached.
Mfg# E7507 C# 699587
Mfg# E7507-DB C# 164418
Communication Type:
Allocation Notification/Update
V#10255
-
08/26/2025
Title: Medtronic V#10255
Details:
Medtronic EEA with DST Technology Discontinuation Customer Letter_Approved 8-25-2025
Medtronic EEA™ circular stapler with DST Series™ Product Notification 8-25-2025
Vendor Item List Sales History with Units --- 2.26.25-8.26.25
Concordance Team,
The Medtronic EEA™ circular stapler with DST Series™ technology products will be discontinued on February 28, 2026. The suggested replacement products, EEA™ circular stapler with Tri-Staple™ technology, are referenced in the attached Excel file.
- Please review the attached customer notification letter for additional details.
- A six-month customer usage report is attached.
- See the discontinued mfg item numbers with cross to Concordance items below:

Communication Type:
Discontinuation Notice
V#10255
-
08/25/2025
Title: Health-O-Meter V#10075
Details:
Are Your Clients’ Wheelchair Scales MDE-Compliant?
The U.S. Access Board’s updated Medical Diagnostic Equipment (MDE) standards are now in effect—and they directly impact wheelchair scales. These changes are a prime opportunity to engage with healthcare facilities and ensure they’re equipped with compliant, accessible solutions.
Health o meter® Professional Scales has published a helpful blog that breaks down the new requirements and what facilities need to know.
Read the blog
Share this with your clients and team to spark conversations and drive informed sales!Please reach out to Brett Lucido, Director of National Accounts blucido@homscales.com, (724) 799-4233 if you have any questions or would like more information on Health o meter® Professional Scales certified for ADA compliance products and the new MDE standards.
thanks,
Kelly Virzi
Sales & Marketing Specialist
- 1-708-377-0549
- kvirzi@homscales.com
9500 West 55th Street, McCook, IL 60525-7110 USA
Pelstar, LLC
Health o meter® Professional Scales | Follow Us
weigheasier®
Communication Type:
Updated Supplier Information
V#10075
-
08/25/2025
Title: GE Medical V#10126
Details:
GE_Copy of Clinical Accessories team
Concordance Team,
A GE sales roster is attached. Notice there are three tabs to open. On the tab with phone numbers, some are missing. An updated copy with any additional phone numbers will be shared when GE provides it.
Communication Type:
Sales Roster
V#10126
-
08/24/2025
Title: Attends V#10055
Details:
Vendor Item List Sales History with Units - 2.22.25-8.22.25
Concordance Team,
Attends is updating their shaped pads towards the end of the year.
- Please see the attached flyer for product change details
- No changes to case counts, bag counts, etc.
- Just updating the bags and new claims on the product
Item numbers:
C# 336520 Mfg# SPDPA
C# 336524 Mfg# SPSA
C# 336521 Mfg# SPDRA
A usage file is attached for customer listing.
Communication Type:
Product Changes
V#10055
-
08/24/2025
Title: Curbell V#12402
Details:
map1795e_disposable_spo2_info_sheet
map652c_disposable_lead_wires_brochure
Concordance Team,
See attached product information from Curbell V#12402. The vendor can be contacted for more details for any customers who want to transition to Curbell’s disposable ECG and SPO2 accessories. They believe partnering on these items may help Concordance displace competition and increase margin.
Contact our Item Maintenance team to set up any of these items if needed per customer demand.
Communication Type:
Product Catalog
V#12402
-
08/22/2025
Title: Hollister V#10901
Details:
Pouch upgrade and attached letter. There are no changes to the GTIN, Weight, Dimensions, Price or HCPC. Attached is the letter
Here’s the landing page with all the messaging, FAQ’s, etc.:
CeraPlus™ Pouch Update | Hollister US
Michelle VeNard
Key Account Manager, Channel
2000 Hollister Drive | Libertyville, IL | 60048 USA
c. 404-277-3039
Making a difference in the journey of life
Communication Type:
Product Changes
V#10901
-
08/22/2025
Title: Attends V#10055
Details:
Attends Free Webinar - How To Retail Incontinence
Vendor Item List Sales History with Units for webinar - 2.22.25-8.22.25Concordance Team,
Please see the attached email for the opportunity to participate in an ATTENDS free webinar. A six-month usage file is attached which I referenced to determine the account managers to include.
Communication Type:
Updated Supplier Information
V#10055
-
08/21/2025
Title: Abbott V#10575
Details:
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File 8.21.25 Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Chad Truini
Sr. Business Analyst
Commercial Operations
Abbott Nutrition
3300 Stelzer Road
Columbus, OH 43219
O:
+1 614-624-0239
Communication Type:
Product Inventory Update
V#10575
-
08/20/2025
Title: Thomas Medical V#10462
Details:
New Product Update:
Thomas Medical just started manufacturing an Oocyte Retrieval Needle and I'm reaching out to see if you're interested in carrying this item.
Please click here to learn more about the product. The needle is competitively priced and comes along with the outstanding quality and service you expect from Thomas Medical.
--
Communication Type:
New Product Launch
V#10462
-
08/20/2025
Title: BD V#10125
Details:
Allocation Update E-Z Scrub Removals
Per the attached, the items below have been removed from allocation. An updated customer listing is also attached from Allocations team.
CHS#563197 mfg#371163
CHS#596023 mfg#372053
CHS#392654 mfg#371603
CHS#970061 mfg#971901
Please reach out to Heather Burkett if you have any questions. Thanks,
Communication Type:
Allocation Notification/Update
V#10125
-
08/20/2025
Title: Pfizer V#12114
Details:
Labor Day Holiday Schedule:
Pfizer Pharmaceuticals Labor Day Hemophilia Holiday Schedule 2025
(BeneFIX® , Hympavzi™ and Xyntha®)
Dear Valued Customer,
We realize the importance of communicating our holiday schedule to customers. To support your planning efforts for Hemophilia orders, we are providing the following information for Pfizer operations during the upcoming holiday period. Please review this information closely.
Pfizer will be closed on Monday, September 1, 2025 in observance of Labor Day. Our Hemophilia operations will be impacted as follows:
Pfizer Holiday Shipping Schedule
Orders Received
Expected Delivery By
Thursday, August 28, 2025 before 4:30pm EST
Friday, August 29, 2025
Thursday, August 28, 2025 after 4:30pm EST
Wednesday, September 3, 2025
Friday, August 29, 2025
Wednesday, September 3, 2025
We will return to our standard business schedule on Tuesday, September 2, 2025. As always, Pfizer will make every effort to accommodate emergency needs as they arise.
If you have any questions about this schedule, please contact us at 1-888-440-8100.
Sincerely,
Kathy Dolan
Director, Customer ServiceCommunication Type:
Holiday Schedule
V#12114
-
08/20/2025
Title: B Braun V#10508
Details:
Allocation Update; Removals for August with Updated listing.
Please see below from B Braun and let me know if you have any questions.
We have exciting news to share today as we were able to take all IV Fluid codes off of allocation. This includes the S8004/S5104 codes that you had to order weekly. As of today, please order as much product as is needed for your DCs with no restriction.
I attached the customer notification letter, the new list of items left on allocation for B. Braun, and your updated August Allocation file. Attached is also an updated customer listing from the allocations team.
Please let me know if you have any questions.
Communication Type:
Allocation Notification/Update
V#10508
-
08/20/2025
Title: ICU V#10917
Details:
Allocation Update; Direct Only Items now Available through Distribution Update
Please see attached email from ICU Medical. These items are now available and IM is in the process of removing from “Direct only”. Allocations have been removed as well.


Communication Type:
Allocation Notification/Update
V#10917
-
08/20/2025
Title: BD V#10125
Details:
Allocation Removals; E-Z Scrub Items
Per the attached from BD, the items below have been removed from allocation. Please see attached customer listing from the allocation team.
CHS#563197 mfg#371163
CHS#596023 mfg#372053
CHS#392654 mfg#371603
CHS#970061 mfg#971901
Please let me know if you have any questions, thanks!
Communication Type:
Allocation Notification/Update
V#10125
-
08/20/2025
Title: ICU V#10917
Details:
Allocation Removals/ Update to Direct Only Items- Now Available through Distributors
Please see below from ICU on items that are now available through Distributors. Thanks,


Communication Type:
Allocation Notification/Update
V#10917
-
08/20/2025
Title: B Braun
Details:
Allocation Update from B. Braun
Please see email below on Allocation update from B Braun.
We have exciting news to share today as we were able to take all IV Fluid codes off of allocation. This includes the S8004/S5104 codes that you had to order weekly. As of today, please order as much product as is needed for your DCs with no restriction.
I attached the customer notification letter, the new list of items left on allocation for B. Braun, and your updated August Allocation file. The allocations team also provided an updated customer listing.
Please reach out to Heather Burkett if you have any questions. Thanks,
Communication Type:
Allocation Notification/Update
V#10508
-
08/20/2025
Title: Pfizer V#12114
Details:
Updated Holiday Schedule
Pfizer Pharmaceuticals Labor Day Hemophilia Holiday Schedule 2025
(BeneFIX® , Hympavzi™ and Xyntha®)
Dear Valued Customer,
We realize the importance of communicating our holiday schedule to customers. To support your planning efforts for Hemophilia orders, we are providing the following information for Pfizer operations during the upcoming holiday period. Please review this information closely.
Pfizer will be closed on Monday, September 1, 2025 in observance of Labor Day. Our Hemophilia operations will be impacted as follows:
Pfizer Holiday Shipping Schedule
Orders Received
Expected Delivery By
Thursday, August 28, 2025 before 4:30pm EST
Friday, August 29, 2025
Thursday, August 28, 2025 after 4:30pm EST
Wednesday, September 3, 2025
Friday, August 29, 2025
Wednesday, September 3, 2025
We will return to our standard business schedule on Tuesday, September 2, 2025. As always, Pfizer will make every effort to accommodate emergency needs as they arise.
If you have any questions about this schedule, please contact us at 1-888-440-8100.
Sincerely,
Kathy Dolan
Director, Customer ServiceCommunication Type:
Holiday Schedule
V#12114
-
08/20/2025
Title: BD V#10125
Details:
Labor Day Schedule:
To help effectively plan your order fulfillment needs, enclosed is a copy of BD’s 2025 Holiday Schedule.
Kindly note the following:
Labor Day (Monday, 9/1)
- BD fully closed (no Customer Care coverage will be available)
- No deliveries and no rush orders will be processed
- Milk Runs from Sparks, MD normally scheduled to ship Monday, 9/1 will depart Saturday, 8/30
- Milk Run shipments normally delivered Monday, 9/1 will deliver Tuesday, 9/2
- No temperature sensitive Parcels will be shipped on 8/29 or 9/1

Communication Type:
Holiday Schedule
V#10125
-
08/19/2025
Title: Verde Enviromental V#12386
Details:
We are going to be Discontinuing the XL Pouch. Letter Attached
Your group hasn't had any sales on this product in the last several months, so there shouldn't be much to do.
Please let me know if you have any questions. I'm working on updating sell sheets.
Have a great day.
_____________________________
Jake Byrnes| Distributor Sales Manager
Deterra, Verde Environmental Technologies, Inc.
12900 Whitewater Dr. #200 | Minnetonka, MN 55343
Mobile 218-220-1062
Communication Type:
Discontinuation Notice
V#12386
-
08/18/2025
Title: Ecolab V#10929
Details:
Ecolab Discontinuance Reminder - August 2025
Vendor Item List Sales History with Units - 2.15.25-8.14.25
Concordance Team,
Effective October 1, 2025, Ecolab is discontinuing the below list of items.
Ecolab introduced NEW Endure™ Plus and Endure™ Plus Sensitive Foam Hand Soaps in December 2025 and will be transitioning all NEXA Endure™ customers to the Endure™ Plus product line by October 1st 2025. The change from Endure™ to NEW Endure™ Plus Foam Hand Soaps requires no change in dispensing equipment and will maintain the same price per push as legacy Endure™.
Their formal communication is attached and can be shared with end users. You may also refer to Alex’s email below.
A 6-month usage report is attached.



Communication Type:
Discontinuation Notice
Vendor #10929
-
08/18/2025
Title: Geri-Care V#12008
Details:
Concordance Team,
See attached and below from Geri-Care V#12008:

Communication Type:
Holiday Schedule
V#12008
-
08/18/2025
Title: Medegen V#10423
Details:
Medegen email_disco item #82830
Vendor Item List Sales History with Units - 2.18.25-8.17.25
Concordance Team,
Medegen will no longer sell mfg #82830 (C#132773) effective immediately per the attached notification. They listed mfg#82830B as a replacement which is C#419928.
One customer appeared in the 6-month usage report which is attached.
Communication Type:
Discontinuation Notice
V#10423
-
08/18/2025
Title: Abbott V#10575
Details:
We are sharing with all of our partners the attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File 8.14.25 Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Communication Type:
Product Inventory Update
V#10575
-
08/18/2025
Title: s2s V#10006
Details:
Good morning,
Please see attached for an important update regarding product availability and order fulfillment on S2S Global products.
Effective immediately, we are temporarily implementing an allocation on all incontinence SKUs to ensure fair distribution of our limited inventory among our valued customers and recommend you continue to order your standard weekly/monthly usage for other products. Our supply teams are closely monitoring inventory and order volumes to support normal order patterns and will be sending out communications if there are any expected supply disruptions. We are committed to being a part of the solution and providing updates as additional information evolves.
Thank you for your continued collaboration and support.
For any questions or concerns regarding your order please reach out to orders@s2s-global.com and/or your sales representative.
Thanks,
S2S Global
7201 Iron Horse Blvd
North Richland, TX 76180
Communication Type:
Allocation Notification/Update
V#10006
-
08/18/2025
Title: SC Johnson V#12219
Details:
Copy of Nationwide soap recall list_SOME HAVE SC JOHNSON SUBS
Vendor Item List Sales History with Units - 2.18.25-8.18.25
Concordance Team,
In response to a Nationwide soap recall of Dermarite products in the attached file, SC Johnson is suggesting their product #626461 (C#263121) as a substitute. If converting a customer to the substitute SC Johnson product, they would need a new dispenser which SC Johnson will provide at no charge to the customer. See Don Allegretti's email below.
Nationwide Soap Recall Issued Over Contamination Linked to Sepsis - Newsweek

Communication Type:
Updated Supplier Information
V#12219
-
08/18/2025
Title: Medtronic V#10255
Details:
Medtronic Tri-Staple 1.0 Customer Discontinuation Letter Approved WF 18001094 07.09.25
Copy of Medtronic 1.0 Tri-Staple™ Reload Product Notification
Vendor Item List Sales History with Units - 2.18.25-8.18.25
Concordance Team,
Effective May 1, 2026, Medtronic will discontinue the 1.0 Tri-Staple™ reloads and offer the 2.0 Tri-Staple™ reloads as alternatives.
Attachments:
The suggested replacement products with acquisition prices are referenced in Medtronic's attached Excel file.
- The cross-reference to Concordance item numbers have been added to the file.
- Five (5) of the items being discontinued are currently set up.
- Of those five (5) items, the suggested replacement codes have not yet been set up.
- Review the attached customer notification letter for additional details.
- Review the attached 6-month usage file for customers who purchase these items.
Thanks,
Tricia
Communication Type:
Discontinuation Notice
V#10255
- The cross-reference to Concordance item numbers have been added to the file.
-
08/14/2025
Title: NDC V#10030
Details:
Triamcinolone INJ Market Update
Concordance Team,
The attached communication is from NDC regarding Triamcinolone 40mg 10ml viles and the supply outlook amid a growing nationwide market outage of triamcinolone injection product from multiple manufacturers. We source the 400mg from NDC, not the 40 mg 10ml viles of Triamcinolone. The 40mg SKUs in VAI come from McKesson and Cardinal. The attached sales report revealed very minimal sales of just one item from McKesson that meets the description in the notification.
Communication Type:
Product Update
V#10030
-
08/12/2025
Title: Ansell V#11003
Details:
Ansell Healthcare Products, LLC
----
111 Wood Avenue South
Iselin, NJ 08830
United States
T. + 1 732 345 5400
F. + 1 732 219 5114
www.ansell.comAugust 11, 2025
On August 1, President Trump announced the implementation of new or updated previously-paused tariffs on goods from multiple countries/regions, several of which Ansell manufactures in. The tariffs range from 10% to 50% and went into effect on August 7.
These new tariffs—combined with broader cost pressures outlined below that are already affecting our global operations—are now driving a need to adjust pricing. In an effort to reduce administrative activities on behalf of our customers, we will implement a single price increase to address current market conditions, effective October 1. Pricing for end-user contracts will also be updated accordingly, effective November 1. Prices will increase based on their relevant tariff and inflationary impacts; overall we anticipate a weighted average increase of approximately 8.5% depending on your mix of purchases. Please ensure to review price books once received for specific increase amount by style, as well as to see exclusions.
We recognize this year has already brought multiple pricing changes and the coordination that comes with them. To limit further disruption, and barring any unforeseen developments, we do not anticipate an additional increase on January 1.
Cost Drivers
Several key factors are being felt across the supply chain and are already impacting costs today, including:- Rising labor costs across global manufacturing regions, particularly in Asia, where general inflation and stronger compliance with social responsibility standards have driven wages upward. As part of our commitment to ethical and responsible labor practices, Ansell maintains high standards across our supply chain, which are reflected in our cost structure.
- Higher energy and utility costs, especially in Asia, where electricity and water expenses remain elevated across key production hubs.
- Global transportation challenges, with sustained increases in ocean freight rates due to Red Sea disruptions, port congestion, and lower carrier reliability—all of which have driven up container and shipping costs.
- Ongoing investment in supply continuity, including the qualification of alternate manufacturing sites outside high-tariff regions, which requires time, regulatory compliance, and upfront cost. These efforts strengthen our long-term cost structure while reducing exposure to future volatility.
In response, Ansell is taking a long-term approach, focused not just on navigating short-term pressures, but on maintaining the standards our customers expect. We continue to prioritize supply continuity, product quality, and ethical sourcing as we adapt to shifting trade conditions —supported by years of investment in a resilient, diversified global network. While some suppliers may respond to cost pressures by lowering product quality or shifting to lower-cost sources, we remain committed to delivering consistent, high-performing solutions without compromise, and are here to support you in lowering total cost of ownership and care delivery through services like AnsellGUARDIAN ®, and tools designed to improve efficiency, reduce risk, and enhance productivity.
Price Change Implementation Details
New pricing will go into effect on October 1, 2025 for all impacted styles. In early September, the pricing team will begin the process of sending out price books that are inclusive of all updates, aiming to share them with all customers by the third week in September.- Increases will apply only to orders placed on October 1, 2025 and forward.
- Increases are in addition to any previously communicated tariff related increases.
- You do not need to take action to approve any new pricing. You do, however, need to ensure your documentation reflects the price changes; please update your system accordingly.
- To ensure a seamless implementation we ask you to please ensure this communication is circulated within your purchasing and operations teams so that all relevant stakeholders are aware.
Finally, while this communication focuses on upcoming pricing changes, we know customers also rely on visibility into product lifecycle updates. We will be announcing any strategic product discontinuations later this year, following a comprehensive, real-time review of the market, portfolio performance, and customer needs.
In the meantime, we will continue to closely monitor market conditions and will adjust our approach as necessary. Should you have any questions, please don’t hesitate to reach out to your Ansell Channel Manager, Territory Manager, or Inside Sales Representative. We appreciate your ongoing partnership and look forward to working together through these evolving circumstances.
Kind Regards,
Sean Sweeney
Chief Commercial Officer, AmericasCommunication Type:
Tarriff
V#11003
Communication Type:
pricing_changes
V#11003
-
08/8/2025
Title: Kawasumi V#11675
Details:
Announcement Of New K-Shield Winged Blood Collection Set
Please see below items which will be discontinued on September 1st. Attached is also a notification letter from the supplier.
Also attached is a customer listing with usage for the past 6 months.
Please reach out to Heather Burkett if you have any questions. Thanks,DBM Series to CBM Series Cross Reference: C# Product
CodeQty/UOM Case
DimensionsCase
WeightProduct
CodeCase
DimensionsCase
Weight769474 D3KA-21G 50 each/box,
10 boxes/case24”x12”x7” 7.4lbs CBM-21G 16”x15”x14” 10.4lbs 207770 D3KA-23G 50 each/box,
10 boxes/case24”x12”x7” 7.4lbs CBM-23G 16”x15”x14” 10.4lbs 207771 D3KA-25G 50 each/box,
10 boxes/case24”x12”x7” 7.4lbs CBM-25G 16”x15”x14” 10.4lbs 200271 DBMA-21G 50 each/box,
4 boxes/case10”x7”x10” 3.2lbs CBMA-21G 12”x14”x8” 4.4lbs 200272 DBMA-23G 50 each/box,
4 boxes/case10”x7”x10” 3.2lbs CBMA-23G 12”x14”x8” 4.4lbs 200273 DBMA-25G 50 each/box,
4 boxes/case10”x7”x10” 3.2lbs CBMA-25G 12”x14”x8” 4.4lbs 203540 DBMH-21G 50 each/box,
2 boxes/case16x13x7 3.2lbs CBMH-21G 16.5x11x6.5 3.439lbs Communication Type:
Discontinuation Notice
V#11675
-
08/8/2025
Title: Ansell V#11003
Details:
Good afternoon-
Attached
is a communication regarding a label update on sku# TS-10, TS-SM, TS-SS, TS-PD, TS-RD. There is no change to packaging, price or UOM. Can you please make sure this gets to the appropriate team? Please let me know if you have any questions.Thanks
Communication Type:
Product Flyer
V#11003
Communication Type:
Product Changes
V#11003
-
08/8/2025
Title: Integra Life V#10275
Details:
The attached letter serves as notification of a voluntary medical device correction for MediHoney® Wound & Burn Products distributed by Integra LifeSciences due to a potential breach in packaging causing a sterility issue. Please note that a fedex overnight mail went out to you on August 5, 2025, with the same letter.
Reason for Communication
This voluntary correction has been initiated due to a potential breach in packaging which creates a sterility concern. The impacted products are listed in Table 1 in the attached letter.
Our records indicate that you may one or more of the impacted products.
Therefore, please take the actions noted below:
Actions to be Taken by Customers (Medical Facility):
- Complete the Medical Facility Acknowledgement Form in the letter. Alternatively, you can use the fillable form titled “Medical Facility Acknowledgement Form, MediHoney Products.
- If you do have units of the impacted product (Table 1) remove them immediately from service and quarantine them.
- If you have affected product, check the box “I do have affected product”. Record the product ID and the total quantity of the affected product.
- If you do not have affected product, check the box, “I do not have affected product.”
- Forward this notice to those who utilize the product so they are aware of the recall and can identify any affected product that ma remain in clinical areas.
- Return the Medical Facility Acknowledgement Form to FCA2@integralife.com or FAX to 1-609-750-4220.
- Keep a copy of the form for your records.
OR
Actions to be Taken by Distributors:
- Complete the Distributor Acknowledgement Form in the attached letter. Alternatively, you can use the fillable form titled “Distributor Acknowledgement Form, MediHoney products.
- If you have products listed in Table 1 remove the product from further distribution.
- 3. If you have affected product, check the box “I do have affected product”. Record the product ID and the total quantity of the affected product.
- If you do not have affected product, check the box, “I do not have affected product.”
- Complete the entirety of the Acknowledgement Form and send via email to FCA2@integralife.com or FAX to 1-609-750-4220.
- Keep a copy of the form for your records.
- Check your customer traceability records and notify them if they have any shipments of above catalog numbers.
- Modify the Acknowledgement Form to create one from you to your customers.
Thank you and please let me know if any questions.
Kind regards,
Mary O’Neill
Director, Post Market Surveillance
Integra Lifescience
Communication Type:
Recall Notification
V#10275
-
08/8/2025
Title: Abbott V#10575
Details:
Attached
you will find the standard weekly inventory update letter you have been receiving:- Abbott Institutional Inventory Letter
- Customer File 8.7.25 Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Chad Truini
Sr. Business Analyst
Commercial Operations
Abbott Nutrition
3300 Stelzer Road
Columbus, OH 43219
O:
+1 614-624-0239
Communication Type:
Product Inventory Update
V# 10575
-
08/8/2025
Title: Graham-Field V#10228
Details:
Greg Lange
Strategic Account Director
GF Health Products, Inc.
1 Graham Field Way, Atlanta, GA 30340
Atlanta GA 30340-3140
M: 770-630-5734
Click HERE to book time on my Calendar
Communication Type:
Product Flyer
V#10228
-
08/8/2025
Title: MicroCare V#10221
Details:
We are officially launching our new Pre-Treatment Gel on August 1st.
For your convenience, I've attached an image of the product and our technical data sheet.
UOM
12oz Can
12CN/CS
Let me know if you need anything else.
FYI - I will send a separate email formatted for your rep email blast.
Thanks,
Charlie Campbell
Key Account Manager
6120 East 58th Avenue
,
Commerce City
,
CO
,
80022
Communication Type:
Product Flyer
V#10221
Communication Type:
New Product Launch
V#10221
-
08/8/2025
Title: Microcare V#10221
Details:
Attached
is all of the product data for our rebrand. This includes product names, descriptions, etc.Please note, the item numbers did not change.
Also included is a Dropbox link for all of the new images.
Please let me know if you have any questions.
Thanks,
Charlie Campbell
Key Account Manager
6120 East 58th Avenue
,
Commerce City
,
CO
,
80022
Communication Type:
Product Changes
V#10221
-
08/8/2025
Title: American Biotech V#12222
Details:
Dear Valued Partner,
We would like to inform you that our previous Ultra Low Temperature (ULT) freezer models are being discontinued. As of June 27th, we officially launched our new ULT freezer line, now featuring an intuitive touchscreen interface, eco-friendly natural refrigerants, and a modernized design.
Please begin phasing out the discontinued models listed below. If you haven’t yet reviewed the new product line, you can access all marketing materials here: ABS Materials
Models to be Discontinued:
· ABT-115V-1786
· ABT-230V-1786
· ABT-115V-2186
· ABT-230V-2186
· ABT-230V-2586
Thank you for your attention to this update and for your continued partnership.
Best regards,
ABS Marketing
Communication Type:
Discontinuation Notice
V#12222
-
08/8/2025
Title: Nestle V#10183
Details:
Per Nestle’s earlier announcement, MCT Oil is being discontinued. Effective August 15th the product will no longer count toward the minimum order quantity on direct orders.
Thanks
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Experience our Support Site
<image001.png>
Communication Type:
Discontinuation Notice
V#10183
-
08/6/2025
Title: Kimberly Clark V#10765
Details:
Kimberly Clark-Customer Portal 8.5.25_Disco list
Kimberly Clark-Customer Portal 8.5.25_Disco mfg 45888
Vendor Item List Sales History with Units - 2.5.25-8.5.25
Concordance Team,
Kimberly Clark has discontinued a group of items in the attached letter.
Mfg #93060 (C#129449) is the only item with usage in the past six months. Usage detail is below.
This letter came from the supplier’s portal, and it states that they included an alternative product list, but this was not actually provided. We have asked the supplier to verify if there is a substitute for this particular item for which we have had small customer demand.
Account Manager ID
Account Manager Name
Lisa
Customer ID
Bill To Name
Customer Item ID
Mfg Stock Number
Mfg Code Description
VAI Item
Item Description
Sell UM
Unit Price
Order Qty
Ship Qty
Sales
Vendor ID
Vendor Name
1023
Calliott, Lisa
ORLA
M361961
SINAI ACUTE CARE LLC
93060
KIMBERLY CLARK PROFESSIONAL
129449
SANITIZER INSTANT HAND GEL 8OZ
EA
73.53
25
15
$367.65
10765
KIMBERLY CLARK PROFESSIONAL
Communication Type:
Discontinuation Notice
V#10765
-
08/5/2025
Title: Coloplast V#10581
Details:
Concordance - DAOH12527 - Mio Black Launch - Oct 1st - For Customer
Concordance CP Distributor Product Load File_Mio Black 07.31.25
Concordance Team,
MESSAGE FROM COLOPLAST V#10581: Coloplast will be launching 34 new ostomy items effective 10/1/25. These are an extension of our SenSura Mio in Black product line. Attached is item set-up information as well as pricing for the following:
- DAOH12527: Acquisition pricing- Exhibit A
- DAOH12527ALT: Alt-Site chargeback pricing- Exhibit A-1
- DAOH12527NON: HME/DME courtesy chargeback pricing- Exhibit A-2
The Item Maintenance team has this information. Contact them if any items need set up due to customer demand.
Communication Type:
New Product Launch
V#10581
-
08/5/2025
Title: United Metal Fabricators V#11120
Details:
The LibertyPRO goes live in the next couple of days.
Lindsay sent out all the pricing information a couple of weeks ago.
We’ll now have 2 different series, Freedom & Liberty, that meet the new https://2418108.fs1.hubspotusercontent-na1.net/hubfs/2418108/Supplier%20Relations/FusionFREEDOMbrochure.pdf.
The new recommendation does require having rails(notice we don’t call them arms)that move so a patient can independently transfer from a wheelchair using the opposite rail to assist.
Highlights of LibertyPRO
650 lb weight capacity
Offering enhanced support rails/TruComfort+(can raise or lower for BP positioning)
Manual or power back versions
Highlights of FusionFREEDOM
450 lb weight capacity
Offering TruComfort Support Rails
Manual or Power Back versions
Eric Estes
Business Development Specialist
UMF Medical | Certified WBENC
C. 828.200.3793
F. 814.266.1870https://calendly.com/eestes-umfmedical/schedule-a-call
This email and any attachments to it are confidential and intended solely for the use of the individual or entity to whom they are addressed. If you are not the intended recipient of this email and/or have received this email in error, please notify the sender immediately, and then delete it. You do not have permission to use, disclose, distribute, forward, or copy this email without the written permission of the author.
Communication Type:
Product Flyer
V#11120
Communication Type:
New Product Launch
V#11120
-
08/5/2025
Title: Medline V#10605
Details:
Medline Backorder Update
Please see below from Medline regarding C#396214. I have included a customer listing with usage for the past 6 months.
Hello Distributor partners,
We now have confirmed recovery dates for MDS001005C – Medline Castile Soap Concentrate. Most branches should receive stock within 3 weeks or sooner.
Please reach out to Heather Burkett with any questions. Thanks,
Communication Type:
Backorder Notification/Update/Reports
V#10605
-
08/5/2025
Title: ICU V#10917
Details: current Ansell rep roster and share with your appropriate teams.
Communication Type:
Sales Roster
V#11003
-
08/5/2025
Title: Medtronic V#10255
Details:
Medtronic Shiley Flex Adult Trach SKU Transition Information Sheet July 2025
Medtronic Distributor List Price - Shiley Cuffed and Cuffless H and A Codes
Vendor Item List Sales History with Units - 2.5.25-8.4.25
Concordance Team,
See attachments and below from Medtronic V#10255 regarding Shiley™ flexible adult tracheostomy tubes with disposable inner cannulas.
- The attached Medtronic Excel file lists the items being discontinued plus replacement item numbers.
- From Medtronic’s attached letter:
- The product reference codes indicating a disposable inner cannula will transition from ending in H (xxCNxxH, xxUNxxH) to ending in A (xxCNxxA, xxUNxxA). There are no changes to the product reference codes for the Shiley™ flexible adult tracheostomy tube with reusable inner cannula (xxCNxxR, xxUNxxR). (Table 1)
- On the flange of the tracheostomy tube, the updated product reference code ending in A (xxCNxxA, xxUNxxA) will replace the code ending in H (xxCNxxH, xxUNxxH). (Table 2)
- The outer cannula inner diameter (I.D.) and the cuff resting diameter will now be etched onto the pilot balloon as an additional reference for clinicians. (Table 3)
- Product packaging has been updated. These updates include new product reference codes and expanded labeling. (Table 4)
- Instructions for Use (IFU) will include additional languages and expanded labeling.
- The planned date of the SKU transition is September 1, 2025, at which time manufacturing of xxCNxxH and xxUNxxH will cease. However, they will remain available alongside their direct replacement xxCNxxA and xxUNxxA through December 31, 2025, or until stock is depleted. We cannot guarantee stock of xxCNxxH or xxUNxxH after September 1, but xxCNxxA and xxUNxxA should be treated as direct replacements.
- Updates will not impact the clinical usage of the product. Clinicians should refer to packaged device labeling for detailed information on products, features, and design when choosing a device for patient use.
- Usage report/customer listing is attached.
Communication Type:
Discontinuation Notice
V#10255
Communication Type:
Label Changes
V#10255
-
08/4/2025
Title: Aspen SurgicalV#10234
Details:
We want to provide an important update regarding recent U.S. trade policy changes and their impact on Aspen Surgical's product portfolio. The newly announced tariffs have led to a notable increase in costs, particularly for finished goods and raw materials sourced from international suppliers. Attached you will find additional information
We appreciate your understanding as we navigate the changing tariff environment and broader market dynamics. To continue providing you with high-quality products, Aspen Surgical will be adjusting prices on select items starting September 1, 2025. We kindly request that you update your pricing system to reflect the prices listed in the attached document. This will help ensure that all orders align with this updated information and prevent any discrepancies.
We value our partnership with you. If you have questions regarding this notification, please contact AspenSurgicalPricing@aspensurgical.com or your Aspen Surgical Sales Representative.
Bradley Liske
CHANNEL PARTNER AND OEM SALES MANAGER
Bradley.liske@aspensurgical.com
PHONE / +1 616.536.75686945 Southbelt Dr. SE, Caledonia, Michigan 49316 USA
aspensurgical.com
Aspen™ Bard-Parker® Bookwalter® Bovie® Olsen® Precept® Symmetry®
Communication Type:
Tarriff
V#10234
-
08/4/2025
Title: Nestle V#10183
Details:
Product Code Changes
Please find attached details for important product updates.
If you have questions, please contact your sales representative for more information.
You may also send questions to HCNContractProcessing@us.nestle.com.
Thank you,
Sales Operations – Contract Management MS
“Unparalleled passion for nourishing and enhancing lives”
Nestlé HealthCare Nutrition, Inc., 11095 Viking Drive, Suite 420, Eden Prairie, MN 55344
T +1 952 848 6204
Communication Type:
Product Update
V#10183
Communication Type:
Product Changes
V#10183
-
08/4/2025
Title: Nestle V#10183
Details:
Nestle inventory letter for this week
- Benecalorie is on the report
- Beneprotein packets is still on the report
- Boost Chocolate was added to the report
- Compleat Original 1.5 250 ml is still on the report
- Boost GC Max 30 gm Protein Vanilla is still on the report
6 Boost GC Max 30 gm Protein Chocolate is still on the report
- Boost Soothe Strawberry Kiwi is still on the report
- Impact Peptide 250 and 1000 ml are still on the report
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Experience our Support Site
<image001.png>
Communication Type:
Product Inventory Update
V#10183
-
08/1/2025
Title: Abbott V#10575
Details:
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File 7.31.25 Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Chad Truini
Sr. Business Analyst
Commercial Operations
Abbott Nutrition
3300 Stelzer Road
Columbus, OH 43219
O:
+1 614-624-0239
This communication may contain information that is proprietary, confidential, or exempt from disclosure. If you are not the intended recipient, please note that any other dissemination, distribution, use or copying of this communication is strictly prohibited. Anyone who receives this message in error should notify the sender immediately by telephone or by return e-mail and delete it from their computer.
Communication Type:
Product Inventory Update
V#10575
-
08/1/2025
Title: Midmark V#10798
Details:
Please see attached Midmark Medical 2026 Price Notification and Price File.
Thank you.
Designing better care.®
Midmark Corporate Accounts Department
T: 1.800.MIDMARK
midmark.com
Communication Type:
pricing_changes
V#10798
-
08/1/2025
Title: QUIDEl/NDC#10030
Details:
Q3 Incentives
we are running with NDC – Market Match Below:Promo, flyers, sales roster
I am also attaching the Customer Buy Get Promos, along with our field roster.
Let me know if you need anything at all!
Sincerely,
Lisa Palmer
Distribution Partnerships Manager
9975 Summers Ridge Road, San Diego, CA 92121
- 800-874-1517 | C. 401-824-9539
Communication Type:
End User Promo
V#10030
Communication Type:
Sales Roster
V#10030
-
07/29/2025
Title: Nestle V#10183
Details:
Attached is the Nestle inventory letter for this week
- Beneprotein packets is still on the report
- Compleat Original 1.5 250 ml
- Boost GC Max 30 gm Protein Vanilla is still on the report
- Boost GC Max 30 gm Protein Chocolate is still on the report
- Boost Soothe Strawberry Kiwi is still on the report
- Impact Peptide 250 and 1000 ml are still on the report
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Experience our Support Site
Communication Type:
Product Inventory Update
V#10183
-
07/29/2025
Title: Medtronic V#10255
Details:
Medtronic Mulitfire US Discontinuation Customer Letter 07.17.25 Approved WF 17593673_
Medtronic MultiFire Premium Skin Stapler Discontinuation Notice
Vendor Item List Sales History with Units - 1.29.25-7.28.25
Concordance Team,
Medtronic sent the below email about four items being discontinued which is also explained in the attached letter.
- Six-month usage report is attached for customer listing by account manager.
- Notice for mfg #059037 & #059038, the discontinuation date in the past.
- Per the letter, mfg #059035 and #059036 will no longer be available after 4/30/2026 or earlier if inventory is depleted sooner.

Some subs are already loaded for these items and others shown below will be loaded.

Communication Type:
Discontinuation Notice
V#10255
-
07/29/2025
Title: GOJO V#10354
Details:
GOJO email - 2 discontinued items
Concordance Team,
Please see below from GOJO regarding these two items that are discontinued:
Mfg#5306-04 C#149449
Mfg#2845-12 C#270965
No customer orders appeared in the 6-month usage report. The account manager and customer relations are being notified via email about a current customer order for mfg#5306-04 (C#149449) that needs canceled with the customer because the item is no longer available.
Communication Type:
Discontinuation Notice
V#10354
-
07/29/2025
Title: PDI V#10497
Details:
Hello Team,
Please see attached and below on new product launch from PDI V#10497. Sani-HP1 wipes. Items have been set up in the system and are available for your customer needs. If you have any questions, please reach out to our Rep David Antebi at the contact information below. I will post information in our Supplier Relations Communications and Notices landing page in Connections
David Antebi
National Accounts Manager - Acute Care
Cell: 973-207-7977
As Always, thank you for all you do.
--------------------------------------------------
Mary Reinhart | Supplier Relations, Manager
Concordance Healthcare Solutions - Tiffin, OH
419-455-2162 | mreinhart@concordancehs.comPositively Impacting Lives
www.concordancehealthcare.comFrom: Tara Davis <tdavis@concordancehs.com>
Sent: Wednesday, July 16, 2025 9:50 AM
To: Mary Reinhart <mreinhart@concordancehs.com>
Subject: FW: HP-1 New Product RequestHi Mary,
Per your request, the following PDI Sani-HP1 Germicidal Wipes have been set up in the system.
S#417067 Mfg #P62572
S#417062 Mfg #P42672
S#417064 Mfg #P83184
S#418092 Mfg #P0125P
--------------------------------------------------
Tara Davis | Item Maintenance Specialist
Concordance Healthcare Solutions - Tiffin, OH
419-455-2044 | tdavis@concordancehs.comPositively Impacting Lives
www.concordancehealthcare.comFrom: David Antebi <David.Antebi@pdihc.com>
Sent: Tuesday, April 8, 2025 10:25 AM
To: Mary Reinhart <mreinhart@concordancehs.com>
Subject: HP-1 New Product RequestCaution: This email originated from outside of Concordance. DO NOT click on links or open attachments unless you were expecting the email, recognize the sender, and know the content is safe. When in doubt, contact the helpdesk.
Hi Mary,
It was great meeting you in person last week at the Concordance NSM!
Following up on our conversation regarding HP-1, I’d like to start the process of loading the information into your system so we’re prepared for the launch.
Please let me know which forms I need to complete to get this process started. Thank you!
Best Regards,
David Antebi
National Accounts Manager - Acute Care
Cell: 973-207-7977
The information in this email is confidential and may be legally privileged. It is intended solely for the addressee. Access to this email by anyone else is unauthorized. If you are not the intended recipient, any disclosure, copying, distribution or any action taken or omitted to be taken in reliance on it, is prohibited and may be unlawful. Because email can be altered electronically, the integrity of this communication cannot be guaranteed. In no event will this email be construed to constitute a binding agreement. Please consider the environment before printing.Communication Type:
Product Flyer
V#10497
-
07/28/2025
Title: Owens&Minor V#12264
Details:
Holiday Closure
We would like to inform you that Owens & Minor Distribution Centers will be closed on Monday, September 1 in observance of the Labor Day holiday.
Please note:
If you normally receive your orders on Mondays unless previous arrangements have been made, all deliveries will be made on your next scheduled delivery day following the holiday. We ask that you adjust your orders accordingly.
Reach out to your Customer Service Team by end of day on Friday, August 22, 2025 to request alternative arrangements. The Customer Service Team will work with the shipping DC to determine if the request can be accommodated. Additional fees may be applied.
If no special delivery is requested and arranged, all freight and fees for off-day deliveries will apply.
Thank you for your partnership and have a safe holiday!
Sincerely,
Kumar Pushkal | Vice President, Distribution Operations
Owens & Minor | Products & Healthcare ServicesCommunication Type:
Holiday Schedule
V#12264
-
07/28/2025
Title: Health-o-Meter V#10075
Details:
Tariff Notification of price increase
I'm writing to inform you of carefully considered pricing adjustments for our Health o meter® Professional scales and Bridge Healthcare Safe Patient Handling product lines.
As the new tariff environment that emerged in April begins to settle into a more stable and predictable pattern, it has become increasingly clear that - despite our best efforts to adjust operations and work with material suppliers on cost-reduction initiatives - we will not be able to fully mitigate or absorb the impact of these tariffs. This leaves us with few options but to offset a portion of the cost increases we have already absorbed and expect to continue facing.
Effective 90 days from now on 10/25/2025, we will implement new pricing that reflects an average increase of approximately 5% to 6%, depending on the product category.
This decision was made only after thorough analysis and consideration. It is necessary to ensure we can continue providing the high-quality products and responsive service you've come to expect from us.
Please review the attached spreadsheet for updated pricing details. We kindly ask that you confirm receipt of this message and the attachment by replying to pricing@homscales.com. If you have any questions, please reach out to Brett Lucido at blucido@homscales.com.
We sincerely value your partnership and appreciate your understanding as we take these steps to maintain our standards of excellence and reliability. Thank you for your continued support—we look forward to our ongoing collaboration in serving our shared end-users.
Best regards,
Ken Harris
President & CEO
Pelstar, LLC Pricing
- 1-800-815-6615
- pricing@homscales.com
9500 West 55th Street, McCook, IL 60525-7110 USA
Pelstar, LLC
Health o meter® Professional Scales | Follow Us
weigheasier®
Communication Type:
Tarriff
V#10075
-
07/25/2025
Title: B Braun V#10508
Details:
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File 7.24.25 Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Chad Truini
Sr. Business Analyst
Commercial Operations
Abbott Nutrition
3300 Stelzer Road
Columbus, OH 43219
O:
+1 614-624-0239
Communication Type:
Product Inventory Update
V#10575
-
07/25/2025
Title: Health-0-Meter V#10075
Details:
Bridge Healthcare by Pelstar Introduces New BridgeAIR™+STAR Kits for More Efficient Patient Care
Pelstar LLC continues to expand Bridge Healthcare’s Safe Patient Handling portfolio with the launch of new BridgeAIR™+STAR Kits. Each kit includes a BridgeAIR™ Air Transfer & Turning Mattress and a high-performance BreatheDRY™ absorbent pad—offering a comprehensive solution that supports safe patient handling, pressure injury prevention, and moisture management. Some kits also include positioning wedges to further assist with offloading protocols. By combining essential products into a single easy-to-place kit, BridgeAIR™+STAR Kits reduce setup time, simplify inventory, and help staff deliver more efficient, consistent care.
For more information on BridgeAIR™+STAR Kits and Bridge Healthcare’s full range of product solutions, visit https://bridgehcusa.com/safe-patient-handling/bridgestar/ or reach out to Brett Lucido, Director of National Accounts at blucido@homscales.com
thanks,
Kelly Virzi
Sales & Marketing Specialist
- 1-708-377-0549
- kvirzi@homscales.com
9500 West 55th Street, McCook, IL 60525-7110 USA
Pelstar, LLC
Health o meter® Professional Scales | Follow Us
weigheasier®
Communication Type:
New Product Launch
V#10075
Communication Type:
Product Flyer
V#10075
-
07/25/2025
Title: Molnlycke V#10008
Details:
Hibiclens 8oz (57508) Bottle - Temporary Packaging July 2025
Vendor Item List Sales History with Units 1.25.25-7.24.25
Concordance team,
Please see the below notification about a TEMPORARY change to the packaging of the 8-oz bottle being used for HibiClens mfg #57508 (C#219477) from Molnlycke V#10008.
From: Bob Perry <Bob.Perry@molnlycke.com>
Sent: Thursday, July 17, 2025 11:28 AM
Subject: Temporary Packaging Update for HibiClens 8 oz Bottle - Distributor/Customer NotificationDear Valued Distribution Partner,
This message is to inform you of a temporary change in the packaging of our HibiClens 8 oz bottle (SKU 57508). Due to an overstock of retail bottles, we have repurposed these containers to ensure uninterrupted supply to our customers.
What’s Changing:
- The bottle will remain the same, but it might come individually boxed. (See images below)
- The volume remains the same—each bottle still contains 8 oz of product.
- Most importantly, the HibiClens solution inside is unchanged. You will receive the same trusted formula that meets all quality and safety standards.
This change is only for a limited time and does not affect the efficacy, formulation, or intended use of the product. We appreciate your understanding and flexibility as we take this step to maintain consistent availability.

Attached is our formal customer notification that you can share with your team and keep on file.
If you have any questions or concerns, please don’t hesitate to contact your Molnlycke Channel Management partner or our customer service team at (800) 843-8497 or email at customer.orders@molnlycke.com.
Sincerely,
Chris Barys
Bob Perry
Director, US Channel Management
Mölnlycke Health Care
Tel 949-306-0709
Web: www.molnlycke.com
Communication Type:
Product Update
V#10008
-
07/24/2025
Title: Medegen V#10423
Details:
Vendor Item List Sales History with Units 1.23.25-7.22.25
Concordance Team,
Please see below from Medegen that mfg #88000 (C#707170) is obsolete effective immediately. Running a 6-month usage report only produced the following:

Medegen listed mfg#88001 as a replacement, but it is not currently set up in our system. Marianna Snavely is checking if we have current subs for this item to list in VAI.
Communication Type:
Discontinuation Notice
V#10423
-
07/24/2025
Title: Kenvue V#10860
Details:
Johnson's Baby Items_with CHS cross ref numbers
Vendor Item List Sales History with Units - 1.23.25-7.22.25
Concordance Team,
Please see the following from Kenvue Brands V#10860 about changes to Johnson’s Baby formulas and packaging per the below email:
“Please see the attached marketing material you can post and share with your sales Team on the changes to Johnson’s Baby formulas and packaging.”
“The changes will flow through beginning in August.”
“There are no changes to price, item, UPC, or GTIN.”
Kenvue also confirmed the following:
- No changes to the carton packaging
- No changes to the quantity per carton
Communication Type:
Product Update
V#10860
-
07/24/2025
Title: Edwards LifeSciences V#10340
Details:
enableCV Action Letter and FAQ_2.10.25
Edwards LS spun off enableCV item list_2025
Edwards Lifesciences Vendor Item List Sales History with Units 1.9.25-7.8.25
Concordance Team,
Edwards Lifesciences V#10340 sold off their EnableCV business and formed it as a separate company called EnableCV. However, per the below message from EnableCV, Concordance will no longer purchase the items because they will be selling the products direct to customers and not through our distribution channel as a result of low sales volume.
- Item Maintenance will inactivate the item numbers on the attached list.
- A usage report from the past nine months is attached---shows sales to only two customers.
Marianna verified that we do not currently have a direct replacement of these items to list as subs. Medtronic has similar items but not the same size. The end user would need to select a product that they are comfortable using.
Communication Type:
Discontinuation Notice
V#10340
-
07/24/2025
Title: Essity V#10272
Details:
Essity Discontinuation Notice
Please see attached notification from Essity/ V#10272
on the discontinuation of two codes and their replacements.C#642267/ 67660
C#642270/ 67670
Attached is also a customer listing
with sales and units purchased in the past 6 months. Please reach out if you have any questions. Thanks,Communication Type:
Discontinuation Notice
V#10272
-
07/24/2025
Title: Audit Microcontrols V#12466
Details:
New Product Launch
On Thursday, 7/17/2025, we are launching a new product, K830M-5 Linearity LQ Ammonia/Alcohol for Ortho Vitros. This will not be available until Thursday, so please make a note. The distributor price for this product is $240.00.

Communication Type:
New Product Launch
V#12466
-
07/22/2025
Title: Medegen V#10423
Details:
7.16.25 Medegen email_Disco item mfg#108MP
Concordance team,
Medegen mfg#108MP (C#222707) has been discontinued and replaced with #108MBX (C#215279). IM has placed C#222707 in item activation.
There has been NO usage for the past 9 months.
Communication Type:
Discontinuation Notice
V#10423
-
07/22/2025
Title: Baxter V#10797
Details:
Allocation Update_EM Inlets_UV Bags_SW_IVsJuly 2025_vF
Concordance team,
Baxter V#10797 announced some recent allocation changes in the attached letter. Some elements of the letter do not pertain to products Concordance distributes.
The items below were increased on our side and the only customers needing notified are below. The other items we either don’t have set up or were already not listed as allocated so no additional changes were needed.
CHS#112511 H938173 125%
CHS#112510 H938174 75%
CHS#110970 H938175 125%
CHS#113796 H938176 125%

Communication Type:
Allocation Notification/Update
V#10797
-
07/22/2025
Title: Mozarc V#13599
Details:
Mahurkar Discontinuation Letter - U.S. Update
Mozarc V#13599 disco item list_cross to CHS #
Vendor Item List Sales History with Units 1.22.25-7.21.25
Concordance Team,
Per the attached Mahurkar Discontinuation Letter from Mozarc Medical V#13599, they are discontinuing their Legacy Mahurkar line of catheters. Mozarc came out with an upgraded version called MAHURKAR ELITE a couple of years ago and the majority of the hospitals have converted. For any facilities still using legacy Mahurkar, Mozarc will officially discontinue by the end of the 2025 calendar year (based on inventory).
Communication Type:
Discontinuation Notice
V#13599
-
07/21/2025
Title: Health-O-Meter V#10075
Details:
Bridge Healthcare’s Gold Rollboards — Your Lightweight Advantage in Patient Transfers
Give your customers a smarter, safer alternative to traditional metal transfer boards. The Gold Rollboard is a lightweight, non-metal patient transfer solution designed to reduce caregiver strain, enhance patient comfort, and support infection control standards— all while being up to 75% lighter than traditional boards.Key Benefits:
➤ Up to 75% lighter than traditional metal boards to minimize staff fatigue
➤ Soft foam core provides optimal patient comfort
➤ Smooth, roller-free, low-friction design ensures gentle patient transfers
➤ Antimicrobial, heat-sealed seams for top-tier infection control
➤ MRI-safe, 100% metal free for use in MRI, and imaging departments
The Gold Rollboard is available in 7 sizes, including foldable models for fowler position transfers to fit a wide variety of departments and clinical needs. From the OR to the ICU, the Gold Rollboard protects staff, preserves patient dignity, and streamlines patient care. For more information, visit https://bridgehcusa.com/gold-rollboards/ or reach out to Brett Lucido, Director of National Accounts at blucido@homscales.com or (724) 799-4233.
thanks,
Kelly Virzi
Sales & Marketing Specialist
- 1-708-377-0549
- kvirzi@homscales.com
9500 West 55th Street, McCook, IL 60525-7110 USA
Pelstar, LLC
Health o meter® Professional Scales | Follow Us
weigheasier®
Communication Type:
Product Flyer
V#10075
-
07/21/2025
Title: Health-O-Meter V#10075
Details:
exciting new announcement from the Bridge Healthcare line to be added to your system. I have attached your Item Add Spreadsheet, Spec Sheet, and images regarding the new BridgeAIR™+STAR Kits. Please reach out with any questions or if I can provide any additional information.
SKU
Description
Cost
MSRP
PS40SPULTB
BridgeAIR™+STAR-LT, Mattress Single Patient Use Full, 40”x80” w/ BreatheDRY™ 37” x 56" Protection Pad
$535.00
$879.00
PS40SPULTSB
BridgeAIR™+STAR-LT Mattress Single Patient Use Half, 40” x 49” w/ BreatheDRY™ 48” x 39” Protection Pad
$440.00
$722.00
PS50SPULTB
BridgeAIR™+STAR-LT Mattress Single Patient Use Full, 50” x 80” w/ BreatheDRY™ 48”x 39” Protection Pad
$373.00
$612.00
PS37SPUOB
BridgeAIR™+STAR-O Mattress Single Patient Use Full, 37” x 56” w/ BreatheDRY™ 37” x 56" Protection Pad
$450.00
$739.00
PS37SPUOBW
BridgeAIR™+STAR-O Mattress Single Patient Use Full, 37” x 56”, BreatheDRY™ 37” x 56" Protection Pad, and BridgeSTAR™ Turning/ Repositioning Wedge with Tail
$330.00
$543.00
Bridge Healthcare by Pelstar Introduces New BridgeAIR™+STAR Kits for More Efficient Patient Care
Pelstar LLC continues to expand Bridge Healthcare’s Safe Patient Handling portfolio with the launch of new BridgeAIR™+STAR Kits. Each kit includes a BridgeAIR™ Air Transfer & Turning Mattress and a high-performance BreatheDRY™ absorbent pad—offering a comprehensive solution that supports safe patient handling, pressure injury prevention, and moisture management. Some kits also include positioning wedges to further assist with offloading protocols. By combining essential products into a single easy-to-place kit, BridgeAIR™+STAR Kits reduce setup time, simplify inventory, and help staff deliver more efficient, consistent care. For more information on BridgeAIR™+STAR Kits and Bridge Healthcare’s full range of product solutions, visit www.bridgehcusa.com.
thanks,
Kelly Virzi
Sales & Marketing Specialist
- 1-708-377-0549
- kvirzi@homscales.com
9500 West 55th Street, McCook, IL 60525-7110 USA
Pelstar, LLC
Health o meter® Professional Scales | Follow Us
weigheasier®
Communication Type:
Product Flyer
V#10075
Communication Type:
New Product Launch
V#10075
-
07/20/2025
Title: J&J V#10196
Details:
JJMT_Surgifoam_Customer Letter_Supply Update_JUNE 2025
Vendor Item List Sales History with Units 1.21.25-7.20.25
Concordance Team,
The allocations have been removed in our system for the following J&J item codes:

Communication Type:
Allocation Notification/Update
V#10196
-
07/18/2025
Title: Abbott V#10575
Details:
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File 7.17.25 Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Chad Truini
Sr. Business Analyst
Commercial Operations
Abbott Nutrition
3300 Stelzer Road
Columbus, OH 43219
O:
+1 614-624-0239
This communication may contain information that is proprietary, confidential, or exempt from disclosure. If you are not the intended recipient, please note that any other dissemination, distribution, use or copying of this communication is strictly prohibited. Anyone who receives this message in error should notify the sender immediately by telephone or by return e-mail and delete it from their computer.
Communication Type:
Product Inventory Update
V#10575
-
Communication Type:
Sales Roster
V#11003
-
07/16/2025
Title: V#10030
Details:
we just launched with Chembio Diagnostics. We have been working with Bob Gannon (included in on this email) and he mentioned that he had previously worked with you in the past to get their product line setup within Concordance. We do now offer the product through NDC that would give you ability to purchase the products through NDC.
Chembio Diagnostics, Inc. – BIOSYNEX Group
NDC Warehouse Program
We enable longer and healthier living through detection and monitoring of infectious diseases at the point of care.
Chembio Diagnostics, a subsidiary of Biosynex SA, develops easy-to-use POC tests for infectious diseases like HIV and Syphilis. Using proprietary industry-leading technology, their tests deliver rapid, accurate results within 15 minutes from fingertip blood or other small samples. Headquartered in Medford, NY, the company serves healthcare providers worldwide.- Small Sample Size: 2.5 μL – 10 μL
- Results in 15 Minutes
- 24-month shelf life at room temp
- Easy to use
- Cost-effective dual reimbursement for HIV-Syphilis
- Made in USA
- Oral fluid testing available with DPP HIV 1/2
- 99.99% HIV sensitivity and specificity
Advin COVID-19 Antigen Test @Home, 1 test/kit
Advin COVID-19 Antigen Test @Home, 2 tests/kit
Advin COVID-19 Antigen Test @Home, 25 tests/kit
DPP HIV-Syphilis Promo Pack, 400 tests (20 kits; 20 tests/kit)
DPP HIV-Syphilis Promo Pack, 700 tests (20 kits; 35 tests/kit)
DPP HIV 1 / 2 Assay, 20 tests/kit
DPP HIV 1/ 2 Rapid Test Control Pack
DPP HIV-Syphilis Control Pack
DPP HIV-Syphilis, 20 tests/kit
DPP Micro Reader, for use w/ HIV-Syphilis
HIV 1 / 2 STAT-PAK Assay, 20 tests/kit
HIV Rapid Test Control Pack (for SURE CHECK and STAT-PAK)
SURE CHECK HIV 1 / 2 Assay, 25 tests/kit
NDC Program Details:
NDC Warehouse Program
Available to the US Only
Available to NDC Medical, MedPlus & Physical Rehab
Markets: Physician/Clinic, Acute Care/Surgery Center, Hospital, Laboratory, Home Health Care, Government, Emergency – EMS, Other Market(s) (list): Student Health, Women’s Health, OB/GYN, Urgent Care, Community Health, Family Planning, Public Health, Federally Qualified Health Centers, Correctional Facilities
CPT Codes / Reimbursement: 86703: Antibody HIV 1 and HIV-2, single result; 86780: Antibody Treponema Pallidum (Syphilis); 87811-QW: Covid-19
National Average Reimbursement: 86703: $13.71, 86780: 13.24, 87811-QW: $41.38
Contact: Robert Gannon, (203) 402-9669
Additional Resources:
Visit Chembio’s website
Download Advin COVID-19 Antigen Test Sell Sheet
Download Chembio’s Rapid Test Sell Sheet
Download DPP HIV Brochure
Download DPP HIV-Syphilis Brochure
Download SURE CHECK HIV Brochure
Download STAT-PAK HIV BrochureCommunication Type:
Product Flyer
V#10030
Communication Type:
Product SKU List
V#10030
-
07/16/2025
Title: Midmark V#10987
Details:
Due to sourcing constraints, several components in the current Wireless PCBA board used in our 626 wireless controls have become obsolete. Midmark is actively developing a solution and targeting October 2nd, 2025 as the implementation date for the updated 626 wireless PCBA board.
As a result, we are implementing a 15-week extended lead time for all new orders for wireless models 626-003 and 626-004, effective immediately. By implementing this lead time now, we are minimizing the impact to current orders and the disruption to customers.
We currently have a backlog (orders in-house) of 98 ea. wireless 626-003/004 models that will be shipped as booked. Any new orders for wireless 626s that are received today will be booked past October 2nd, 2025.
As we get closer to the implementation date, we anticipate the extended lead time to shorten. At this time, we do not anticipate any opportunities pushing out of 2025 into 2026.
Updates will be provided as more information becomes available.
Regards,
CONFIDENTIALITY NOTICE: This message, including any attachments, contains confidential information intended for a specific individual and purpose. If you are not the intended recipient, you should delete this message and any disclosure, copying, or distribution of this message, or the taking of any action based on it, by you is strictly prohibitedCommunication Type:
Backorder Notification/Update/Reports
V#10987
Communication Type:
Supply Disruption
V#10987
-
Communication Type:
Updated Supplier Information
V#12264
-
07/16/2025
Title: Nestle V#10183
Details:
Attached is the Nestle inventory letter for this week
- Beneprotein packets is still on the report
- Boost Chocolate was added to the report
- Boost GC Max 30 gm Protein Vanilla was added to the report
- Boost GC Max 30 gm Protein Chocolate was added to the report
- Boost Soothe Strawberrry Kiwi is still on the report
- Impact Peptide 250 and 1000 ml are still on the report
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Experience our Support Site
Communication Type:
Product Inventory Update
V#10183
-
Communication Type:
supplier_marketing_campaign
V#10078
Communication Type:
Label Changes
V#10078
-
07/15/2025
Title: V#10008
Details:
Dear Distributor Partner: letter attached
Mölnlycke regrets to inform you that we will be unable to fill orders of Mepore Film 4” x 10” (manufacturer part number 272500) until Q2 2026. Please note, this is the only SKU of the Mepore Film line that is affected.
Other Mepore Film sizes may be potential alternatives for your facility. Please find ordering information for the available codes below:
Product Code
Product Description
Pcs/Box
Pcs/Case
271500
Mepore® Film 4" x 5" (10 x 12.7cm)
70
210
273000
Mepore® Film 6" x 8.5" (15 x 21.5cm)
10
50
We apologize for any inconvenience during this period and will update you as the product becomes available. Attached is our formal notification of this supply situation for your files.
Please contact your Mölnlycke sales representative with any questions.
Sincerely,
Bob
Bob Perry
Director, US Channel Management
Mölnlycke Health Care
Tel 949-306-0709
Web: www.molnlycke.com
Communication Type:
Discontinuation Notice
V#10008
-
07/14/2025
Title: V#10575
Details:
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File 7.10.25 Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Chad Truini
Sr. Business Analyst
Commercial Operations
Abbott Nutrition
3300 Stelzer Road
Columbus, OH 43219
O:
+1 614-624-0239
Communication Type:
Product Inventory Update
V#10575
-
07/10/2025
Title: ICU V#10642
Details:
Legacy Smith Medical Discontinuation Notice:
Please see discontinuation notification from ICU Medical. Please see Customer Usage file here. (ONLY Blanchard Valley).
Please reach out to Heather Burkett if you have any questions. Thanks,
Communication Type:
Discontinuation Notice
V#10642
-
07/10/2025
Title: B Braun V#10508
Details:
JULY Allocation Updates:
Solutions Allocation Increase letter
1000mL_Nutrilipid_Ltr_FINAL_063025
B Braun Allocation File- July 2025
Please see below and attached from B Braun. This is the July Allocation file which also includes information on whether allocation increases are allowed for each product code.
Attached is also a customer listing with current allocation amounts.
- All items highlighted in green were increased to 120% allocation and referenced in attached customer letter – Solutions Allocation Increase Letter- increased to 120% (table below)
- We added Nutrilipid (S4600/S4601/S4603) highlighted in orange has been added to allocation – 1000mL Nutrilipid Ltr- these items were already allocated on our side/ no updates
- We removed Meropenem 1g (NDC 00264-3185-11) off allocation- removed from allocation
R8005
00264738650
;6/19 120% inc
250509
L7501
00264775010
;6/19 120% inc
207613
L8001
00264780010
;6/19 120% inc
446666
L8002
00264780020
;6/19 120% inc
459727
L5100
00264751000
;6/19 120% inc
463976
R8206
00264738860
;6/19 120% inc
209611
L6100
00264761000
;6/19 120% inc
878678
L6120
00264761200
;6/19 120% inc
349779
L6350
00264763500
;6/19 120% inc
878496
L6520
00264765200
;6/19 120% inc
876482
L7070
00264770700
;6/19 120% inc
147258
L7510
00264775100
;6/19 120% inc
349647
L8500
00264785000
;6/19 120% inc
400895
Q8020
00264580400
;6/19 120% inc
409895
S8506
00264738560
;6/19 120% inc
282529
S8705
00264738750
;6/19 120% inc
255189
V4500
00264917020
6/19 INCREASE TO 110%
441972
L7511
00264775110
;6/19 120% inc
596130
Q8001
00264580210
;6/19 120% inc
360419
L6250
00264762500
;6/19 120% inc
302600
L6340
00264763400
;6/19 120% inc
300497
L6450
00264764500
;6/19 120% inc
110299
L7301
00264773010
;6/19 120% inc
196684
R8306
00264738960
;6/19 120% inc
222981
S8505
00264738550
;6/19 120% inc
255187
P8721
00264987210
;6/19 120% INC;6/23 (23) LEN REBALANCING
380063
L5100
00264751000
6/18 (34)DS;6/19 120% inc
463976
L6100
00264761000
6/18 (119)DS;6/19 120% inc
878678
L6120
00264761200
6/18 (22)DS;6/19 120% inc
349779
L6350
00264763500
6/18 (5)DS;6/19 120% inc
878496
L7070
00264770700
6/18 (13)DS;6/19 120% inc
147258
L7501
00264775010
6/18 (30)DS;6/19 120% inc
207613
L7510
00264775100
6/18 (35)DS;6/19 120% inc
349647
L8001
00264780010
6/18 (51)DS;6/19 120% inc
446666
L8002
00264780020
6/18 (292)DS;6/19 120% inc
459727
Q8001
00264580210
6/18 (67)DS;6/19 120% inc
360419
R8206
00264738860
6/18 (2)DS;6/19 120% inc
209611
Communication Type:
Allocation Notification/Update
V#10508
-
07/10/2025
Title: Medline V#10605
Details:
Micro Kill Bleach (EVSCHEM910) packaging change
Hello Distributor Partners,
We’re making a packaging update to our Micro Kill Bleach (EVSCHEM910). Starting soon, each case will include 2 trigger sprayers and 2 pour caps instead of 10 each. This change helps reduce waste and creates cleaner packaging.
If you need more sprayers or caps, they’re available for purchase:
EVSCHEM010 – Flip Top Pour Cap (1 ea)
EVS4900H – Trigger Sprayer (1 ea)
Also, we’re moving from corner sticker labels to pre-printed labels for a cleaner, more professional look.
Thanks,
Mansi
Communication Type:
Product Changes
V#10605
-
07/10/2025
Title: Medline V#10605
Details:
O2 Mask Conversion Announcement
O2 Mask Conversion_Customer Letter_June 2025
Customer Listing with Usage and Sales Dollars is attached here.
Hello Distributor Partner,
Medline will begin phasing out its legacy oxygen masks starting mid-Q3 2025, transitioning to the Medline Hudson RCI line. This change supports our commitment to quality, clinical performance, and patient comfort.
What to expect:
Legacy items will be discontinued and replaced with Hudson RCI equivalents.
A conversion chart is included to guide you through replacements.
Some items will be discontinued without direct substitutes, with alternatives noted.
Timeline:
Transition begins: Mid-Q3 2025
Completion: End of 2025
Please review the attached documents for full details.
Communication Type:
Discontinuation Notice
V#10605
-
07/10/2025
Title: B Braun V#10508
Details:
RECALL Notification:
Please see below links for letters on recall notification. If you have any questions please reach out to Jody Dasenbrock. Thanks,
Communication Type:
Recall Notification
V#10508
-
07/10/2025
Title: Essity V#10272
Details:
DISCONTINUATION: (UOM CHANGE)
Please see Customer Listing here.
Please see Distributor Notification here
Please see warehouse guide here
Please reach out to Heather Burkett if you have any questions. Thanks,

Communication Type:
Discontinuation Notice
V#10272
-
07/9/2025
Title: Welch Allyn V#10834
Details:
Baxter WA Exam Tools Promotional Flyer_LR
Baxter WA Q-Stress Promotional Flyer_LR
Baxter WA Resting ECG Promotional Flyer_LR
Baxter WA RetinaVue Price Promotional Flyer_LR
Baxter WA Spot Vision Screener Primary Care Rebate Promo Flyer_LR
Baxter WA Spot Vision Screener Trade-Up Promo Flyer_LR
Baxter WA Vital Signs Promotional Flyer_LR
Concordance Team,
Please see the attached promos from Welch Allyn for Q3.


Communication Type:
End User Promo
V#10834
-
07/8/2025
Title: Zoll Medical V#10276
Details:
BLACKOUT PERIOD
I am writing to notify you about ZOLL’s upcoming blackout period, July 18th – July 31st, while we migrate over to Oracle Cloud in efforts to better serve our customers. During this blackout period, ZOLL will not be able to process any new POs. All POs received before Thursday July 17th will continue to ship in the order that they were received. Ordering is scheduled to resume on Friday, August 1st.
Important Dates
- July 17th – cutoff for PO submission.
- July 18th – July 31st – blackout period, all EDI/webstore/hard copy POs will be on hold.
- August 1st – ordering will resume
Action Requested
- SUPPLY CHAIN: Please place a direct order for 1 months’ worth of your ZOLL consumables prior to Thursday, July 17th. If you already have ZOLL consumables on order, please place another direct order to get ahead of extended lead-times. I’ve attached ZOLL’s latest memos regarding electrode lead-times.
- BIOMED: Place orders for any replacement accessories or parts for upcoming PMs prior to Thursday, July 17th.
Thank you again for your patience and partnership as we work on creating a better ordering experience for our customers.
Jennifer Koenig
Territory Manager – North Los Angeles
ZOLL Resuscitation, Hospital Sales
Cell: 720-301-9114
CONFIDENTIALITY NOTICE: This e-mail communication and any attachments may contain confidential and privileged information for the use of the designated recipients named above. If you are not the intended recipient, you are hereby notified that you have received this communication in error and that any review, disclosure, dissemination, distribution or copying of it or its contents is prohibited. If you have received this communication in error, please notify me immediately by replying to this message and destroy all copies of this communication and any attachments. Thank you.
Communication Type:
Supply Disruption
V#10276
-
07/8/2025
Title: NDC V#10030
Details:
Product Flyers
Download these provider-focused flyers to support your marketing efforts!
Email not displaying correctly?
View it in your browser.Provider Flyers from
Top Manufacturer Partners
Flyers to Support Your Marketing EffortsFeatured Vendors:
Abbott
BinaxNOW PromoAnsell
Q3 PromoBaxter
VisionBD
Veritor PromoBD
Veritor Buy 2 Kits PromoBD
Veritor Digital vs VisualBD
VeritorBD
Veritor Group A StrepBD
Veritor ImageMoverBD
Veritor SARS-COV-2BD
Veritor SynapsysBD
Veritor TriplexDETECTO
apexDukal
Wound CareDuracell
Procell in HealthcareGOJO
SustainabilityGraham Medical
Sea Me GrowHealth o meter
ADA CompliantMedtronic
ShileyMetrex
Easy as 123O&M Halyard
Law Black GlovesO&M Halyard
Tattoo Black GlovesPDI
SaniHP1SEKISUI
Combo TestsSolventum
Wound CareTerumo
SurGuard3 FactsTerumo
SurGuard3 NeedleTerumo
SurGuard 3ZOLL
AED3Be social & follow:
Copyright © 2025, All rights reserved.
NDC, Inc.
402 BNA Drive, Ste. 500
Nashville, TN 37217Communication Type:
Product Flyer
V#10030
-
07/8/2025
Title: Baxter V#10797
Details:
Customer Letter_Discontinuation of Select IV Solution Codes
Vendor Item List Sales History with Units 1.9.25-7.8.25
Concordance Team,
FROM BAXTER V#10797: Subject: Baxter Product Discontinuation Notice
Effective December 31, 2025, Baxter will be discontinuing the products listed in Table 1. These product codes will no longer be manufactured after this effective date. We apologize for any inconvenience this may cause your facility.
Table 1: Product Codes being discontinued on December 31, 2025, Descriptions and NDC Product Code Description NDC
See the usage report that is attached and Baxter's attached letter for additional information.Communication Type:
Discontinuation Notice
V#10797
-
07/8/2025
Title: Bionix V#10641 - New Product Offering
Details:
2025 Bionix Concordance Price List
MKT-0314 (REV-01) - Rechargeable Light Source Sellsheet
Please see the attached and below from Bionix.
Good afternoon,
Bionix is excited to announce the launch of our newest product, the Rechargeable Light Source, an upgrade that our customers have been asking for.
The Bionix® Rechargeable Light Source for ClearLook® Lighted Ear Curette™ enhances cerumen removal with improved visibility, precision and eco-friendly technology. Its rechargeable design reduces waste, while compatibility with familiar and preferred curette tip styles ensures a seamless, cost-effective upgrade for clinicians.
Key benefits:
- USB-C rechargeable with available multi-port charging base
• Crisp LED for superior visibility and precision - Cuts waste from disposable Light Sources - Long-Term Savings
- Refill tips available to reduce reorder costs - Long-Term Savings
• Compatible with all current Bionix ClearLook & Lighted products
Attached is your updated 2025 price list with all applicable SKUs highlighted in yellow for your easy reference. Please visit the Bionix website to learn more or contact me directly if you’d like media assets or additional sales support when launching the new Rechargeable Light Source.
The Rechargeable Light Source and associated SKUs are available and in stock. Please let us know if you have any questions or need additional information to help you get these new items set up in your system.
Thank you,
Sarah Helle
Contract Administrator
New CHS#s are as follows

With any further questions you may have, please reach out to Jeff Shook.
Kind Regards
Communication Type:
New Product Launch
V#10641
- USB-C rechargeable with available multi-port charging base
-
07/7/2025
Title: Medtronic V#10255
Details:
Concordance Team,
Following an announcement from Medtronic V#10255, the allocations have been increased in our system from 90% to 105% items mfg#E7507 (C#699587) and mfg#E7507-DB (C#164418).
Communication Type:
Allocation Notification/Update
V#10255
-
07/7/2025
Title: Ferris Mfg V#10168
Details:
Labeling-Silicone Border Dressing
A letter from Roger Session - IFU Modification
Vendor Item List Sales History with Units 1.7.25-7.6.25
Concordance Team,
SEE ATTACHED 6-MONTH USAGE FILE, THE BELOW MESSAGE FROM FERRIS MFG V#10168 AND THEIR TWO (2) ATTACHED PDF DOCUMENTS:
Dear Distribution Partner, The attached notification concerns important updates to the Instructions for Use (IFU) for our PolyMem Silicone Border Dressings. These modifications have been made to support the most common clinical applications and out of an abundance of caution related to potential ethylene oxide (EtO) sterilization residuals. I have also attached a note from Roger Sessions, CEO, of Ferris Mfg. Corp. addressing the IFU modification.
To ensure clarity and patient safety, we've updated the product labeling as follows:
- Adult Use Only: Product codes REF# 1248, 1249, 2439, 2466, 2468, and 2499 will now be labeled for adults only, and are not for use on young children or neonates.
- Adult and Child Use: Product codes REF# 1243, 1245, 2423, and 2435 will be labeled for use on adults and children but are not for neonates.
For all PolyMem Silicone Border Dressings, regardless of patient age (pediatric or adult), always refer to the product's IFU for complete guidance.
Updated IFUs are included in all product shipments you received after June 25, 2025. You can also access the most current IFUs on the PolyMem website: https://polymem.com/instructions-for-use/.
Please let me know if you have any questions or concerns and I will follow-up with you as soon as I’m able. We appreciate your continued partnership and attention to these updates.
Sincerely,
Rick Otero
Dir of Contracted Sales Team and Distribution Channels
FERRIS MFG. CORP.
Communication Type:
Label Changes
V#10168
-
07/7/2025
Title: Sentec V#12363
Details:
SDM Consumable Discontinuation Letter - Concordance Chicago
Vendor Item List Sales History with Units 1.7.25-7.6.25
Concordance Team,
Please see the attached 6-month usage file for the below discontinuation notice from Sentec V#12363 plus their attached discontinuation letter.
Dear Valued Customer,
In response to U.S. import tariffs and ongoing economic uncertainty, we will discontinue the following lower-volume products effective October 31, 2025. Additional information can be found within the attached letter.

This decision is intended to offset rising costs and shift focus to alternative products with improved usability and support.
We are here to support you and your staff through this change and hope to make this a seamless transition. Please reach out to your local Territory Sales Manager or to Customer Service with any questions.
Communication Type:
Discontinuation Notice
V#12363
-
07/7/2025
Title: J&J V#10196
Details:
EMAIL_J&J 10196 Med Tech Surgery Product Launch-Ethicon 4000 & Ethicon 3D Reloads
Concordance Team,
See attached email from J&J about a new product launch.
Some details from the J&J's email: We would like to notify you that Johnson & Johnson MedTech is introducing the following codes to its
laparoscopic stapling portfolio. The codes will be available for purchase for distributor stocking orders on
August 30, 2025.
ETHICON 4000 is complete system; the ETHICON 4000 stapler codes only work with the ETHICON 3D reloads.
The ETHICON 4000 system codes are not compatible with any legacy codes.Communication Type:
New Product Launch
V#10196
-
07/7/2025
Title: Newman V#11466
Details:
End User Q3 Promo
CURRENT PROMOTIONS - VALID THROUGH SEPTEMBER 30, 2025
DigiDop Doppler Trade-In
- Trade ANY Doppler → Get $100-$200 rebate with purchase of a new DigiDop
simpleABI Trade-In
- Trade ANY ABI device → Get up to $1,500 rebate with the purchase of a new simpleABI Cuff-Link system
- NOTE: Customer must complete redemption form to receive FREE probe (ships separately from Denver): https://newman-medical.com/steadydop-redemption-form/
NEW PRODUCT LAUNCH PROMOTION: The SteadyDop Vascular Probe
- FREE 5 MHz SteadyDop with new Doppler-based simpleABI purchase
- $100 OFF a SteadyDop with DigiDop purchase (now $259.95, reg. $359.95)
- 🔥 SPECIAL OFFER: Can combine with trade-in promotions!
- Visit the SteadyDop vascular probe landing page
- New blog: Why SteadyDop is revolutionizing primary care vascular assessment
Click the graphic below to download the PDF of the promotions.
Find all your DigiDop and simpleABI sales and marketing resources—brochures, sell sheets, cross-references, and more—conveniently located on the Sales Rep Portal.
Copyright (C) 2025 Newman Medical. All rights reserved.
You're our rep!
Our mailing address is:Newman Medical
5350 Vivian Street, Unit C
Arvada, CO 80002
Want to change how you receive these emails?
You can update your preferences or unsubscribeCommunication Type:
End User Promo
V#11466
-
07/7/2025
Title: SC Johnson V#12219
Details:
07.1.25-TruShot 2.0® No Rinse Sanitizer-Annoucement (1)
Concordance Team,
Please refer to the attached document from SC Johnson V#12219 for a new item being launched in August 2025: TruShot 2.0® No Rinse Sanitizer.
Subject: TruShot 2.0® No Rinse Sanitizer
July 1, 2025
Dear Valued Partner
We are pleased to share that we are launching a new addition to our TruShot 2.0® mobile dispensing system. Coming August 2025 is our new TruShot 2.0® No Rinse Sanitizer.
TruShot 2.0® No Rinse Sanitizer is safe on virtually any hard, non-porous surface including food contact surfaces and kills up to 99.99% of foodservice bacteria. With up to 21 in-use quarts in every cartridge, this easy-to-use sanitizer is a great solution for food service facilities, schools, universities, high traffic retail environments, and more. It cleans, sanitizes, and deodorizes in one labor saving step – without the need for rinsing. Paired with a durable trigger sprayer that can be filled at any water source, TruShot 2.0® No Rinse Sanitizer is a time-saving tool designed to keep crews moving.
We encourage you to act now to set up this new SKU in your systems. Taking these steps early will help ensure smooth order processing and allow you to promptly meet the needs of our shared customers when purchase orders are submitted. Together, we can continue to exceed expectations and drive innovation in surface care. Contact your SC Johnson Professional representative today to get started.
Please see linked documents with all the TruShot 2.0® portfolio set up information, inclusive of pricing.
Best,
Wes KlipfelMarketing Manager, Professional Markets
2815 Coliseum Centre Dr. #600
Charlotte, NC 28217
800-248-7190
www.scjp.comCommunication Type:
New Product Launch
V#12219
-
07/7/2025
Title: Nestle V#10183
Details:
Attached is the Nestle inventory letter for this week.
- Beneprotein packets is still on the report
- Boost Chocolate was removed from the report
- Boost Soothe Strawberrry Kiwi was added to the report
- Compleat Pediatric Peptide 1.0 was added to the report
- Compleat Pediatric Peptide1.5 was added to the report
- Impact Peptide 250 and 1000 ml are still on the report
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Experience our Support Site
<image001.png>
Communication Type:
Product Inventory Update
V#10183
-
Communication Type:
Product Flyer
V#10228
-
07/3/2025
Title: Cardinal V#10801
Details:
Wound Care product disruptions
Attached is a recent letter with recovery dates. Several will have extended timelines for recovery.
Let me know if you need Mary Reinhart to identify alternatives.
Communication Type:
Backorder Notification/Update/Reports
V#10801
-
07/3/2025
Title: Medical Action V#10045
Details:
Price Increase letter
At Owens & Minor, we remain committed to delivering high-quality medical products that support healthcare professionals in providing the best possible care for their patients.
This includes our Custom Procedure Trays (CPTs) as well as our Minor Procedure Kits and Trays.
As you are aware, shifting global trade policies and supply chain dynamics are creating widespread challenges across industries, including healthcare.
Over the past several months, we have taken proactive steps to mitigate the impact of these changes such as securing domestic suppliers and optimizing our onshore and near-shore production.
While these efforts have helped, the scale and persistence of global tariffs are now creating cost pressures that can no longer be fully absorbed.
Tariff-Related Price Increase
Effective August 1, 2025, a tariff-related price increase will be applied to both our HALYARD* Custom Procedure Trays and Minor Procedure Kits and Trays.
This will include updates to the list price and end-user agreement prices. The list price changes are attached with this notice. End-user agreement prices will be communicated via the standard process.
Due to the high degree of customization and variability in these products, tariff impacts are unavoidable.
We will continue to closely monitor the global trade environment and will make further pricing adjustments as warranted by changes in tariffs or supply conditions.
Thank you for your continued partnership and trust in Owens & Minor. If you have any questions or would like to discuss the details of these changes, please reach out to your Owens & Minor account representative.
Sincerely,
Andrea Harrison
Senior Vice President, Products & Solutions
Owens & Minor, Inc.Communication Type:
Tarriff
V#10045
-
07/3/2025
Title: American Biotech V#12222
Details:
It was great to speak with you yesterday! I wanted to reach out and pass along some good information for your team regarding our American Biotech Supply cold storage products.
As the Summer months ramp up there is an emphasis put on keeping valuable samples safe in Laboratory/Pharmacy Refrigerators and Freezers.
The ABS product portfolio has your customers covered with our full line of +4°C and -20°C undercounters and uprights.
Attached is a useful flyer for your team in the field breaking down the importance of proper Purpose Built Laboratory/Pharmacy Refrigerators and Freezers.
The large bulk of our products continue to ship in 7-10 business days and our Fast Track Inventory stock is built for some of our highest moving SKUs to ship in 1-2 business days.
Please let me know if there are any questions or opportunities I can help with. I’m always happy to join a team call, provide training for newer reps, and schedule field days with your team out in the field.
Thank you for your support!
Horizon Scientific, Inc.
Jason Guilliam
125 Varnfield Drive
Key Account Manager / Sales Team Lead
Summerville, SC 29483
Office +1.843.821.8010 x 173
Mobile +1.843.737.1932
Fax +1.843.821.8051
Office: 843.821.8010 Customer Service: Option 3 Technical Service: Option 5
Click Here to view Blood and Plasma Refrigerator and Freezer Brochure
Communication Type:
Product Flyer
V#12222
-
07/3/2025
Title: Kate Farms V#12409
Details:
Kate Farms will be raising WAC price on October 1, 2025. This e-mail is the official notification being sent to note this upcoming change. The details by SKU can be found in the attached spreadsheet.
Kate Farms is working to hold down costs - you will see some SKUs are not changing while others are increasing. We are being selective on where WAC pricing is adjusting. This change only reflects the invoice cost you will see when purchasing – it does not have any reflection or change on any pricing contract to customers.
The second attachment is a specific letter regarding how Contract Pricing rebates and chargebacks will be processed in October-2025. Let me know how you would like to proceed.
Please send a reply acknowledging receiving this e-mail.
Let me know if you have any questions about this or need anything further. We thank you for your continued partnership and support.
Regards,
Greg Phipps
Senior Director, Distribution & Partnerships
904-401-3080
katefarms.comCommunication Type:
Updated Supplier Information
V#12409
-
07/3/2025
Title: Avid V#10129
Details:
Dear Valued Customer,
At Owens & Minor, we remain committed to delivering high-quality medical products that support healthcare professionals in providing the best possible care for their patients. This includes our Custom Procedure Trays (CPTs) as well as our Minor Procedure Kits and Trays.
As you are aware, shifting global trade policies and supply chain dynamics are creating widespread challenges across industries, including healthcare. Over the past several months, we have taken proactive steps to mitigate the impact of these changes such as securing domestic suppliers and optimizing our onshore and nearshore production. While these efforts have helped, the scale and persistence of global tariffs are now creating cost pressures that can no longer be fully absorbed.
Tariff-Related Price Increase
Effective August 1, 2025, a tariff-related price increase will be applied to both our HALYARD* Custom Procedure Trays and Minor Procedure Kits and Trays. Due to the high degree of customization and variability in these products, tariff impacts are unavoidable. We will continue to closely monitor the global trade environment and will make further pricing adjustments as warranted by changes in tariffs or supply conditions.
Thank you for your continued partnership and trust in Owens & Minor. If you have any questions or would like to discuss the details of these changes, please reach out to your Owens & Minor account representative.
Sincerely,
Andrea Harrison
Senior Vice President, Products & Solutions
Owens & Minor, Inc.*Registered Trademark or Trademark of O&M Halyard or its affiliates. ©2025. All rights reserved.
Communication Type:
Tarriff
V#10129
-
07/2/2025
Title: Abbott V#10575
Details:
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File 7.2.25 Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Chad Truini
Sr. Business Analyst
Commercial Operations
Abbott Nutrition
3300 Stelzer Road
Columbus, OH 43219
O:
+1 614-624-0239
Communication Type:
Product Inventory Update
V#10575
-
07/2/2025
Title: Stryker V#10912
Details:
7.1.25 Stryker Instruments V#10912 email
Vendor Item List Sales History 1.1.25-7.1.25
Concordance Team,
A usage file is attached for the following:
- After receiving notification from Stryker Instruments V#10912, the following three items have been set to temp unavailable status, and the recommended subs have been applied in the system accordingly.

- Stryker also provided the following information about these mfg item numbers. I have asked for more details so that we know if this will escalate to temp unavailable status in the future.

Communication Type:
Temp Unavailable
V#10912
-
07/1/2025
Title: Baxter V#10797
Details:
Customer Letter_Discontinuation of Select IV Solution Codes
Vendor Item List Sales History 1.1.25-7.1.25
Concordance Team,
See below and the attached letter from Baxter for two items that are being discontinued effective 12/31/25. The usage report is attached for reference.

Communication Type:
Discontinuation Notice
V#10797
-
07/1/2025
Title: HR Healthcare V#11338
Details:
Concordance Team,
See attached images and the following from HR HEALTHCARE V#11338: HR Healthcare is launching a new product in the market called TruLock. This product is a foley securement device that is currently available in a 2-Way and 3-Way product. The Trulock foley securement device is equivalent to the leading BD foley securement device with a HCPCS code of A4333. Please reach out if you should have any questions regarding this product. Once loaded, would you kindly send me a confirmation.
Thank you,

Communication Type:
New Product Launch
V#11338
-
07/1/2025
Title: Kenvue V#10860
Details:
Concordance Team,
See below and attached from Kenvue V#10860 regarding a new product that will be available in September 2025.
New From Listerine! Shipping 9/01/25
Listerine Zero -Cool Mint single use Sachets!
- Perfect for Patient Single use!
- “On the Go” for pocket or purse
- Great for Patient Kits!
- Convenient for Bathroom & Locker Room use
- $0.31 per use!
All information is on the attached PDF. If you have any questions, please reach out to me.
Thank you,
Tynah Hunt
Kenvue Account Manager
609 644 3672
Communication Type:
New Product Launch
V#10860
-
07/1/2025
Title: Attends V#10055
Details:
Comfees Premium Baby Wipes sell sheet.APPROVED.EMAIL
Concordance Team,
See attached sell sheet for new Comfees Unscented Baby Wipes, 72 count.
FROM ATTENDS V#10055: Here is a new item we just launched. Comfees Baby Wipes 72 count non-scented.
PFI- has submitted them to the State of Oklahoma Health Authority to add it to the state formulary. They might be wanting to
buy these.

* Digital Library Link to 3D Images, short and long descriptions below:
* Comfees Premium Baby Wipes sell sheet above for your sales folks.
Communication Type:
New Product Launch
V#10055
-
07/1/2025
Title: GE Healthcare V#10126
Details:
Concordance Team,
See below from GE Healthcare V#10126 regarding their holiday schedule:

Communication Type:
Holiday Schedule
V#10126
-
06/30/2025
Title: Nestle V#10183
Details:
Attached is the Nestle Inventory Letter for this week.
- Beneprotein packets is still on the report
- Boost Chocolate has been added to the report
- Benecalorie was removed from the report
- Compleat Original 1.0 was removed from the report
- Peptamen 1.5 PreBio was removed from the report
- Impact Peptide 250 and 1000 ml are on the report
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Experience our Support Site
<image001.png>
Communication Type:
Product Inventory Update
V#10183
-
06/27/2025
Title: Greiner V#11403
Details:
PRODUCT MODIFICATION Notice
Please see pdf notification here.
Please see customer listing here.
VACUETTE® FX SODIUM FLUORIDE / POTASSIUM OXALATE TUBES
Dear Valued Customer,
We would like to inform you of an exciting upcoming product change.
At Greiner Bio-One, we are dedicated to driving both innovation and sustainability. As a pioneer in the Preanalytics sector, we continuously evolve our product portfolio to better serve your needs in this dynamic and ever-changing market.
As part of our efforts in reducing our product carbon footprint, we have made a change to our VACUETTE® FX Sodium Fluoride / Potassium Oxalate tubes. We have been able to reduce the amount of rubber used in the caps without any change to the piercability of the stopper and there is no effect on vacuum or shelf-life of the VACUETTE® tube.
There is no change in the usual product performance, but please note that there is a minor optical difference regarding the surface below the ring plate as shown in the image below (old cap on the left, new cap on the right).
The following items are affected: Item No. Description Qty. (rack/case)
454238
VACUETTE® Tube 2 mL FX Sodium Fluoride / Potassium Oxalate
13x75, grey cap/white ring, non-ridged
50 / 1,200 454297 VACUETTE® Tube 4 mL FX Sodium Fluoride / Potassium Oxalate 13x75, grey cap/black ring, non-ridged 50 / 1,200
456020
VACUETTE® Tube 5.5 mL FX Sodium Fluoride / Potassium Oxalate
13x100, grey cap/black ring, non-ridged
50 / 1,200
The transition to the new product is scheduled for Q4 2025.
As always, the Greiner Bio-One Technical Services team is available to support you in the use of all our products at (800) 515-8112 or patech@gbo.com. You may also contact Customer Service at (888) 286-3883 or your local Greiner Bio-One Account Manager for assistance.
Best regards from Greiner Bio-One,
Shama Fronczak, BS, MLS (ASCP)CM
Product Manager - PreanalyticsCommunication Type:
Product Changes
V#11403
-
06/27/2025
Title: Medtronic V#10255
Details:
Medtronic Consignee Notification US Appendix A RE00570395_B_EN
Medtronic Consignee Confirmation Form US Appendix B_RE00570395_B_EN
Concordance Team,
Medtronic provided the attached notification and confirmation form for what is described in the below email. However, there is NO USAGE for the past 12 months, so there is no customer listing or usage report included in this posting.


Communication Type:
Product Update
V#10255
-
Communication Type:
New Product Launch
V#10502
-
06/26/2025
Title: Abbott V#10575
Details:
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File 6.26.25 Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Chad Truini
Sr. Business Analyst
Commercial Operations
Abbott Nutrition
3300 Stelzer Road
Columbus, OH 43219
O:
+1 614-624-0239
Communication Type:
Product Inventory Update
V#10575
-
06/25/2025
Title: ICU Medical V#10917
Details:
Recall Notification
Please see below from ICU Medical. If you have any questions, please reach out to Jody Dasenbrock, Recall Coordinator. Thanks,






Communication Type:
Recall Notification
V#10917
-
06/25/2025
Title: Sekisui V#10161
Details:
Dear Valued SEKISUI Diagnostics Partner,
- As a Metrix distributor, we are informing you that we have discontinued the Metrix Reader (Gen 1) effective May 22, 2025.
- Aptitude Medical Systems has developed a second-generation Metrix Reader (P/N# MTRX-RDR-GEN2) for use with the Metrix COVID-19 Test and the Metrix COVID/Flu Test.
- SEKISUI Diagnostics is replacing all the first-generation Readers for our active accounts.
- Please take steps to remove Metrix Reader (Gen 1) (P/N# MTRX-RDR) from your vendor portal.
Please see attached notification here.
This item is dropship only so there is not a customer listing. Please reach out if you have any questions or concerns. Thanks,
Communication Type:
Discontinuation Notice
V#10161
-
06/25/2025
Title: Medline V#10605
Details:
Probe Cover Backorder Update
Customer Listing for the past 6 months is attached.
We are currently experiencing a short-term backorder due to a raw material shortage. As a result, we will not have consistent healthy inventory on the following items until early fall.

However, we do have valid substitute options for each impacted item. Please refer to the chart below for guidance on what to advise your accounts to order in the meantime.

Communication Type:
Backorder Notification/Update/Reports
V#10605
-
06/25/2025
Title: BD V#10125
Details:
Holiday Schedule
Dear Channel Partner,
To help effectively plan your order fulfillment needs, enclosed is a copy of BD’s 2025 Holiday Schedule.
Kindly note the following:
Independence Day (Friday, 7/4)
- BD fully closed (no Customer Care coverage will be available)
- No deliveries
- Milk Runs from Sparks, MD normally scheduled to ship Friday, 7/4 will depart Thursday, 7/3 and deliver on their normal delivery day.
- No temperature sensitive Parcels will be shipped on 7/3 or 7/4.
Thank You,
BD Supply Chain Relations
Communication Type:
Holiday Schedule
V#10125
-
06/25/2025
Title: B Braun V#10508
Details:
Recall Notification
Please see below. If you have any questions or concerns please reach out to Jody Dasenbrock, Recall Coordinator. Thanks,



Communication Type:
Recall Notification
V#10508
-
06/25/2025
Title: Pfizer V#12114
Details:
Holiday Schedule
Pfizer Pharmaceuticals July 4th Hemophilia Holiday Schedule 2025
(BeneFIX® , Hympavzi™ and Xyntha®)
Dear Valued Customer,
We realize the importance of communicating our holiday schedule to customers. To support your planning efforts for Hemophilia orders, we are providing the following information for Pfizer operations during the upcoming holiday period. Please review this information closely.
Pfizer Holiday Schedule
Wednesday, July 2, 2025
Closing at 6:00pm EST
Thursday, July 3, 2025
Closed
Friday, July 4, 2025
Closed
Pfizer Holiday Shipping Schedule
Orders Received
Expected Delivery By
Tuesday, July 1, 2025 by 4:30pm EST
Wednesday, July 2, 2025
Tuesday, July 1, 2025 after 4:30pm EST
Thursday, July 3, 2025
Wednesday, July 2, 2025 by 4:30pm EST
Thursday, July 3, 2025
Wednesday, July 2, 2025 after 4:30pm EST
Tuesday, July 8, 2025
We will return to our standard business schedule on Monday, July 7, 2025. As always, Pfizer will make every effort to accommodate emergency needs as they arise.
If you have any questions about this schedule, please contact us at 1-888-440-8100.
Sincerely,
Kathy Dolan
Director, Customer ServiceCommunication Type:
Holiday Schedule
V#12114
-
06/25/2025
Title: ICU V#10917
Details:
Please see below from ICU, V#10917.
Please see attached customer listing here.
The following Marathon Medical Items are also effected by this- C#374303 and C#397341. Inventory is still available on these items and they will be discontinued once stock is depleted. Thanks,




Communication Type:
Discontinuation Notice
V#10917
-
06/24/2025
Title: Baxter V#10797
Details:
Concordance Team,
From Baxter V#10797: “Effective immediately the following codes have been placed on a protective 100% allocation in response to a market event. I will provide more information as it becomes available.” Customer allocations for the following items were loaded overnight in our system. A customer listing is also attached.

Communication Type:
Allocation Notification/Update
V#10797
-
06/24/2025
Title: Medline V#10605
Details:
Item Backorder Notice - CX102 & CX302 Concordance
PLEASE NOTE this item is not stocked anywhere so there isn't a usage file attached to this notification. Thanks,
This notice is to inform you that item CX102 & CX302 are currently backordered. Anticipated recovery to fill orders is mid-September.
We are able to offer MDS80529 & MDS80529AS as substitute options in the meantime. Please find the table below outlining the product differences.

Communication Type:
Backorder Notification/Update/Reports
V#10605
-
06/24/2025
Title: Medline V#10605
Details:
Allocation update- DYND30224, DYND30228 & DYND30240 on temporary allocation
Medline is seeing high demand on several items that we currently offer as an alternative to our Specimen Collection item, DYND30212, which is on a long term backorder.
Three of our midstream collection kits: DYND30224, DYND30228 & DYND30240 are being placed temporarily on allocation. These were all substitute SKUs for the DYND30212. Allocation quantities are 100% of average recent usage. We are expecting to be healthy again and remove allocation by the end of July.

Overview: DYND30224(H), DYND30228(H) & DYND30240(H) are all midstream collection kits that contain a specimen container, cleansing wipe and patient ID label.
Allocation: These items are being placed on allocation. We expect to be healthy and remove allocation by end of July.
Communication Type:
Allocation Notification/Update
V#10605
-
06/23/2025
Title: Nestle V#10183
Details:
Attached is the Nestle Inventory letter for this week.
- Benecalorie is still on the report
- Compleat Original 1.0 was added to the report
- Peptamen 1.5 PreBio is still on the report
- Impact Peptide 250 and 1000 ml are on the report
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Experience our Support Site
<image001.png>
Communication Type:
Product Inventory Update
V#10183
-
06/23/2025
Title: Sempermed V#10078
Details:
I hope you are having a great week! I wanted to introduce you to a product we have coming soon.
It is a surgical radiation glove. It is a specialized and expensive glove so it will not be a good fit for everyone but I wanted to share it with your reps that are focused on ASC or hospitals.
If you can direct me to your reps that focus in this area we can set up a teams training.
Thank you!!!!
Amy Hattaway | National Accounts Manager
HARPS USA, Inc.
amy.hattaway@harpsglobal.com | Cell: 904.762.8270
www.sempermedusa.com | www.gotgloves.com | www.harpsglobal.com
Communication Type:
Product Flyer
V#10078
Communication Type:
New Product Launch
V#10078
-
06/23/2025
Title: DeRoyal V#10221
Details:
attached a letter that addresses a decision we have made as a company to exit the Custom Tray market. Our local field reps were notified today, and they have begun contacting our end-user tray customers to notify them and offer assistance as they evaluate replacement CPT suppliers. Please let me know if you have any questions about this notification.
Thanks,
Kristen Rogers
Sr. Director – Sales Operations & Distributor Relations
DeRoyal | 200 DeBusk Lane | Powell, TN 37849
email: krogers@deroyal.com
Communication Type:
Discontinuation Notice
V#10221
Communication Type:
Product Changes
V#10221
Communication Type:
Product Update
V#10221
-
06/19/2025
Title: V#10575
Details:
attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File 6.19.25 Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Communication Type:
Product Inventory Update
V#10575
-
06/17/2025
Title: GE HC Amersham V#10301
Details:
6.16.25 GEHC V#10301 Contrast Media FW18 SKU Availability
GEHC Contrast Media FW18 SKU Availability FW24
Concordance Team,
GE Healthcare/Amersham V#10301 provided their updated contrast media SKU listing which is attached. This was converted to Excel and Concordance item numbers have been added where applicable. If there is future customer demand for items not currently set up in our system, please reach out to the Item Maintenance team when set up is required.
VENDOR's EMAIL:
Dear Business Partner,
Please find attached updated GEHC Contrast Media Availability List of SKU’s.
Feel free to reach out to me for your inquiries regarding to our contrast media portfolio.
Thank you for your ongoing support and partnership.
Thank you,
Ryan Dye (he/him/his)
Contrast Media Team Lead
PT Ombuds
GE HealthCare
3350 N Ridge Ave
Arlington Heights, IL 60004 USA
C +1 781-719-8518
O +1 800-292-8514
F +1 866-375-2093
Gehealthcare.com
Communication Type:
Product SKU List
V#10301
-
06/17/2025
Title: Canviiy V#13638
Details:
please see Canviiy attached in the zipped folder. Please review and let us know if you're all set.
When the products are live on the site's catalog, can you please share links?
Thanks for your partnership!
Sherrel
--
WBENC & ACDBE Certified
Sherrel Sampson
Founder & CEO
5004 E. Fowler Ave Ste. C118, Tampa, FL 33617
On Thu, May 29, 2025 at 4:06 PM Sherrel Sampson <ssampson@canviiy.com> wrote:
Communication Type:
Product Flyer
V#13638
Communication Type:
New Product Launch
V#13638
-
06/17/2025
Title: Baxter V#10797
Details:
Concordance Team,
The allocations have been removed from the system for the following four item number per a notification received from Baxter V#10797. The customer listing is attached for your reference.

Communication Type:
Allocation Notification/Update
V#10797
-
06/16/2025
Title: Nestle V#10183
Details:
Attached is the Nestle inventory report for this week
- Benecalorie is on the report
- Compleat 1.5 250 ml are on the report
- Beneprotein packets are on the report
- Peptamen 1.5 Prebio 250 ml are on the report
- Impact Peptide 250 and 1000 ml are on the report
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Experience our Support Site
<image001.png>
Communication Type:
Product Inventory Update
V#10183
-
06/16/2025
Title: Ansell V#11003
Details:
- attached is the info on the new Hybrid Micro product.
Let me know if you have any questions.
Thanks
Communication Type:
New Product Launch
V#11003
-
Communication Type:
Sales Roster
V#11003
-
06/13/2025
Title: Abbott V#10575
Details:
attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File 6.12.25 Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Chad Truini
Sr. Business Analyst
Commercial Operations
Abbott Nutrition
3300 Stelzer Road
Columbus, OH 43219
O:
+1 614-624-0239
This communication may contain information that is proprietary, confidential, or exempt from disclosure. If you are not the intended recipient, please note that any other dissemination, distribution, use or copying of this communication is strictly prohibited. Anyone who receives this message in error should notify the sender immediately by telephone or by return e-mail and delete it from their computer.
Communication Type:
Product Inventory Update
V#10575
-
06/13/2025
Title: Marketlab V#11935
Details:
new promotion on select models of our Insight Carts that I don’t want your sales team to miss out on with their customers. Carts Promo page
Insight Carts deliver durable construction, reliable performance, and intuitive organization and storage. And now your customers can streamline their workflow AND their budget with valuable rebates.
From June 1 through August 31, your customer earns a rebate of $100 or $150 with the purchase of select Insight Ergo Phlebotomy Carts and Storage Carts. Our Marketlab sales team is in the fieldfocusing on this promotion. If there is any way you can reinforce this with your sales team, that would be very beneficial. Feel free to call or email me if you're interested in learning more about our Insight Carts.
For details on the rebates, visit the Carts Promo page on our website.
Very best,
Bob and Jeff
Communication Type:
End User Promo
V#11935
-
06/13/2025
Title: Midmark V#10987
Details:
Please find below links to a new "Revolutionizing Access" podcast and article, sponsored by Midmark and recently published in Repertoire Magazine. Please feel free to share links to the article and podcast with your sales team and the few key takeaways outlined below. The online version of this June issue went live last week, and printed issues should be hitting mailboxes this week. Both include Midmark’s new “We're reaching new heights” ad.
Article: https://repertoiremag.com/revolutionizing-access.html [repertoiremag.com]
What’s in it for your Concordance sales team?
- Lead with compliance: Be first to market with a procedure chair that meets all current US Access Board accessibility standards—no retrofitting or guesswork required.
- Open doors with differentiation: The 631 creates a strategic edge for reps calling on facilities looking to future-proof their equipment.
- Drive cross-sell potential: The 631 pairs perfectly with the Midmark® 626 Exam Chair—creating a solution for exam and procedure rooms that's accessible, ergonomic and compliant.
What’s in it for the Care Team?
- Improved efficiency and ergonomics: With a 22″ of seat height adjustability (17″ low to 39″ high), providers can treat patients comfortably in a standing or seated position.
- Streamlined patient transfers: The chair’s 17" low-seat height and transfer-friendly design reduce the strain on staff and can improve patient flow.
- Patient-centered design: Accessibility accessories like articulating knee crutches and patient support rails as well as design features such as a narrow base compatible with patient lifts reflect thoughtful, real-world clinical needs.
What’s in it for the Patient?
- Accessibility for all: The 17" low seat height accommodates 96% of wheelchair users, making healthcare more inclusive.
- Enhanced safety and comfort: Thoughtful design helps patients feel more secure and respected throughout their visit.
- Future-ready care: With aging and disabled populations growing, accessible equipment is no longer optional—it’s essential.
Thank you and let me know if you have any questions.
CONFIDENTIALITY NOTICE: This message, including any attachments, contains confidential information intended for a specific individual and purpose. If you are not the intended recipient, you should delete this message and any disclosure, copying, or distribution of this message,Communication Type:
Product Flyer
V#10987
Communication Type:
New Product Launch
V#10987
-
06/11/2025
Title: OmnimedV#10625
Details:
We are pleased to announce the launch of Omnimed’s newest product lines, designed to enhance efficiency, safety, and organization in healthcare environments. As a proud American manufacturer, Omnimed remains committed to delivering high-quality products that support workplace safety and effective patient management.
New Phlebotomy Carts
Our latest phlebotomy carts feature ergonomic designs and secure storage solutions, aimed at improving patient care while streamlining clinical workflows.New Acrylic Products
Explore our expanded range of acrylic solutions, including multi-purpose sign holders and durable bulk dispensers—ideal for maintaining hygiene and optimizing workspace organization.For more information or to discuss stocking opportunities, please don’t hesitate to contact us.
Sincerely,
Dan DeCristofer
Dan DeCristofer
Sales & Shipping
Phone. 800-257-2326 X 1131
Web. www.omnimedstore.com
Address. 800 Glen Ave, Moorestown, NJ 08057
Communication Type:
New Product Launch
V#10625
-
06/10/2025
Title: Medline V#10605
Details:
Dyna-Hex CHG Market Update
Please see email below with an update from Medline on their Dyna-Hex CHG line. Attached is a listing of customers who have purchased the below items in the past 6 months.
C#
Medline Part Number
Status
Sub Info
218890
MDS098725
Recovery: End of May
263198
MDS098730
Backorder: Current Recovery: Early August
263195
MDS098708
Healthy
182563
MDS098710
Healthy
160787
MDS098715
Backorder: Late May, Recovery: Mid-June
Sub: Another size of 4% Dyna-Hex CHG (MDS098708)
284416
MDS098731
Healthy
Not set up
MDS098701
Backorder: Early June Recovery: Early Sept.
Direct Sub: ALA57517 (Hibiclens)
Not set up
MDS098719
Discontinued (Notice was sent last market update)
Not set up
MDS098732FM
Backorder: Early June Recovery: Mid-July
Please reach out if you have any questions. Thanks,
EMAIL FROM MEDLINE:
Please review the lastest updates on our Dyna-Hex CHG line.
MDS098725, our 32oz 4% CHG Bottle, is set to fully recover by end of May.
MDS098701, our 15ml Packets, are set to be on an extended backorder due to material delays. See below for more information on the rest of the Dyna-Hex portfolio.
As you may know, Hibiclens (Mölnlycke) has announced the discontinuation of their 32oz (ALA57532) and Gallon (ALA57591) 4% CHG as of July 1st, 2025.
Our MDS098725 and MDS098730 are a direct cross, respectively. Please provide any updates to the demand for these items so we can pivot to the Dyna-Hex due to the Hibiclens discontinuation.
4% Dyna-Hex CHG
SKU
Description
Status
MDS098701
15ml packets, 4% CHG
Backorder: Early June Recovery: Early Sept.
Direct Sub: ALA57517 (Hibiclens)MDS098708
8oz bottle, 4% CHG
Healthy
MDS098710
4oz bottle, 4% CHG
Healthy
MDS098715
16oz bottle, 4% CHG
Backorder: Late May
Recovery: Mid-June
Sub: Another size of 4% Dyna-Hex CHG (MDS098708)MDS098719
18oz bottle, hand-pump foaming 4% CHG
Discontinued (Notice was sent last market update)
MDS098725
32oz bottle, 4% CHG
Recovery: End of May
MDS098730
Gallon, 4% CHG
Backorder: Current
Recovery: Early August
MDS098731
4oz bottle, hand-pump foaming 4% CHG
Healthy
MDS098732FM
32oz round bottle, 4% foaming CHG
Backorder: Early June
Recovery: Mid-July
Thank you,
Julie
Conversation ID: 6b541b60-4307-11f0-8ebc-26fb7c872fea 6b541b60-4307-11f0-8ebc-26fb7c872fea 00c0d40
Communication Type:
Backorder Notification/Update/Reports
V#10605
-
06/10/2025
Title: Carl Zeiss V#11669
Details:
Drape Update:
Please see email below from Carl Zeiss, V#11669.
C#385050 has been replaced with C#415325. Attached is a customer listing for C#385050 with sales history over the past 6 months.
The price file for the drape price changes has been requested and will be updated as soon as possible. Please reach out if you have any questions. Thanks,
EMAIL:
Dear valued Customer,
Thank you for your continued use of the ZEISS KINEVO 900, ZEISS PENTERO 800 S and/or ZEISS TIVATO 700 surgical microscopes.
Please note that a 5% increase on list price of all NES/ETP drapes except SMARTDRAPES 306029 and 306030 went into effect on June 1st, 2025.
With the introduction of our latest Advanced Visualization System - ZEISS KINEVO 900 S, we would also like to inform you that we now have a new ZEISS SMARTDRAPE.
The new SMARTDRAPE 306030 has the same design as ZEISS SMARTRDAPE 306029 and can be used for KINEVO 900 S, KINEVO 900, PENTERO 800 S as well as TIVATO 700*.
Please update your order system and add the ZEISS material number for the new SMARTDRAPE. Details below:
ZEISS Material #
Item Description
For use with:
Action for you:
306029-0000-000
ZEISS SMARTDRAPE
KINEVO 900 S
KINEVO 900
PENTERO 800 S
TIVATO 700*
Discontinue ordering immediately
306030-0000-000 (NEW)
ZEISS SMARTDRAPE
Order item using this material number (306030-0000-000) starting immediately
*Requires laser micromanipulator adapter
Should you require further assistance concerning the price of drapes or technical specifications of the new SMARTDRAPE 30, please contact your local ZEISS Sales team or reach out directly to:
Nimish Tolat
Head of Marketing, Neurosurgery
(925) 413-2557
Ann McCafferty
Head of Marketing, Visualization and Surgical Oncology
(925) 548-5633
Communication Type:
Discontinuation Notice
V#11669
-
06/10/2025
Title: TIDI V#10260
Details:
TIDI - Equipment Covers on Temporary Backorder
Please see attached notification from TIDI regarding 10 sku’s which are temporarily unavailable. I have attached a customer listing for users who have purchased in the past 6 months.
C#
258033
385677
190958
733861
257010
Please reach out if you have any questions. Thanks,
Communication Type:
Temp Unavailable
V#10260
-
06/10/2025
Title: Pfizer V#12114
Details:
Pfizer Juneteenth Holiday Schedule:
Pfizer Pharmaceuticals Juneteenth Hemophilia Holiday Schedule 2025
Dear Valued Customer:
We realize the importance of communicating our holiday schedule to customers. To support your planning efforts for Hemophilia orders, we are providing the following information for Pfizer operations during the upcoming holiday period. Please review this information closely.
Pfizer Holiday Schedule
Thursday, June 19, 2025
Closed
Pfizer Holiday Shipping Schedule
Orders Received
Expected Delivery By
Tuesday, June 17, 2025 by 4:30pm EST
Wednesday June 18, 2025
Tuesday, June 17, 2025 after 4:30pm EST
Thursday, June 19, 2025
Wednesday, June 18, 2025 by 4:30pm EST
Thursday, June 19, 2025
Wednesday, June 18, 2025 after 4:30pm EST
Tuesday, June 24, 2025
We will return to our standard business schedule on Friday, June 20, 2025. As always, Pfizer will make every effort to accommodate emergency needs as they arise.
If you have any questions about this schedule, please contact us at 1-888-440-8100.
Sincerely,
Kathy Dolan
Director, Customer ServiceCommunication Type:
Holiday Schedule
V#12114
-
06/10/2025
Title: ICU Medical V#10917
Details:
Acapella™ DH & DM Respiratory Therapy Systems Supply Disruption Notification
Attached is also a customer listing with end users that have purchased in the past 6 months.
Please reach out to Heather Burkett if you have any questions. Thanks,
Communication Type:
Allocation Notification/Update
V#10917
-
06/10/2025
Title: BD V#10125
Details:
June Allocation Tool and update
Please see attached June allocation tool from BD and notification.
The below have been removed from allocation except for the bold one which was only removed from Temp Unav. Customer listing attached.
Vendor item
Description
% Allocation
Additional Direction
930480
317151
CLEAR SOLUTION/APPLICATOR
100%
Reactivate code June 2 2025
302830
196669
20ML SYRINGE,LUER LOCK
Remove
Remove from allocation June 2, 2025
302995
240059
10ML SYRING,LUER-LOK
Remove
Remove from allocation June 2, 2025
309659
173722
1ML SYRINGE,TB,LUER SLIP
Remove
Remove from allocation June 2, 2025
367341
796128
Wingset PBBCS 25x.75 12 w/ Luer
Remove
Discontinued: Remove from allocation
367352
957027
WINGSET PBBCS 21X.75 12 PREATTACHED
Remove
Discontinued: Remove from allocation
368656
116597
WINGSET PBBCS 23X.75 12 PREATTACHED
Remove
Discontinued: Remove from allocation
368659
167121
WINGSET PBBCS 25X.75 12 PREATTACHED
Remove
Discontinued: Remove from allocation
260100
113347
SWABSTICK,CHLORAPREP,1.75 ML,SINGLE
Remove
Remove from allocation June 2, 2025
1727170102
258688
0.9% Saline 50 mL
Remove
1727170103
258690
0.9% Saline 100 mL
Remove
Communication Type:
Allocation Notification/Update
V#10125
Communication Type:
Temp Unavailable
V#10125
-
06/10/2025
Title: Sekisui V#10161
Details:
SPIFF and END User Promo
Please see end user promo and SPIFF that Sekisui is offering between July 1st- Sept 30th.
- Must be new usage which is tracked through sales tracing reports
- $10 per kit provided
- Payout per Concordance Spiff Compensation Program. Please refer to your Sales Director for details.
Please reach out with any questions and happy selling!
EMAIL from Supplier:
We are happy to announce our 2025 OSOM® Rapid Respiratory Infection Test promotions for end users and rep spiffs starting July 1st!
End-User Promotion:
- This is fulfilled by SEKISUI Diagnostics so there is nothing to do on your end except spread the word to your customers. Flyer is attached for dissemination to both your reps and end users.
- Qualifying products include:
- OSOM® COVID-19 Antigen Rapid Test (cat. no. 1066-40)
- OSOM® Ultra Plus Flu A&B Test (cat. no. 1032)
- OSOM® Flu SARS-CoV-2 Combo Test (cat. no. 1080)
- OSOM® RSV Test (cat. no. 1091)
Rep Spiff:
- Qualifying products include:
- OSOM® Ultra Plus Flu A&B Test (cat. no. 1032)
- OSOM® Flu SARS-CoV-2 Combo Test (cat. no. 1080)
- OSOM® RSV Test (cat. no. 1091)
- The COVID-19 Test does not qualify for the rep spiff.
- To participate, we will need your approval, and you will need to agree to the terms as outlined in the flyer.
- If you would like to participate in the rep spiff, please respond to this email confirming that you accept the terms and let us know which products you’d like to participate with (you can participate with all three products or just one or two).
Please provide a response by June 16, 2025.
- Please “Reply to All” as our Commercial Marketing Team and Channel Sales Team will be managing participation and tracking.
After the end of the rep spiff program, you must submit tracing reports for the programs you elect to participate in. Once tracings are received, a credit memo will be issued so you can pay your reps accordingly.
Please let me know if you have questions.
Communication Type:
Rep Spiff
V#10161
-
06/9/2025
Title: Cardinal V#10801
Details:
Upcoming holiday notice, your Cardinal Health replenishment center will be closed for business on Friday, July 4th.
- Please adjust any ordering that typically falls on Wednesday, 7/2, Thursday, 7/3 and Friday, 7/4 to be ordered on the same specific day a week prior; essentially doubling up on ordering the week of 6/23.
For standing delivery appointments (SDAs), adjustments will be coordinated by your transportation replenishment center leader.
If you have further questions, please contact Customer Service at 800.635.6021. Thank you for your continued business and your cooperation during this holiday time.
Thank you,
Cardinal Health Channel Partners Group
Communication Type:
Holiday Schedule
V#10801
-
06/9/2025
Title: Abbott V#10575
Details:
attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File 6.5.25 Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Chad Truini
Sr. Business Analyst
Commercial Operations
Abbott Nutrition
3300 Stelzer Road
Columbus, OH 43219
O:
+1 614-624-0239
Communication Type:
Product Inventory Update
v#10575
-
06/5/2025
Title: B Braun V#10508
Details:
June Allocation Files
Please see allocation file from B Braun, V#10508 for June. Please let me know if you have any questions.
Updates:
- Attachment labeled, “Current Allocations 6_2_25”, column F- “ARF’s accepted” tells whether B Braun is accepted allocation increases on the item.
- Items listed below have been removed from allocation
- C#101545
- C#191606
- C#194734
- C#315690
- C#460600
Communication Type:
Allocation Notification/Update
V#10508
-
06/5/2025
Title: Medline V#10605
Details:
Discontinuation Notice from Medline, V#10605.
Per the emails below from Mansi @ Medline, S#267584 (Mfg # MDS096160B) has been discontinued and placed into item inactivation. Once inventory depletes in WCST it will inactivate with no suggested replacement
Customer Listing for the past 6 months is attached here.
MDS096160B item being discontinued due to a change in the vendor part number. Please review CTR000410 - MOUTHWASH,ALCOHOL-FREE,16 OZ as a suggested alternate. This is not an exact cross.
Please reach out to Heather Burkett if you have any questions. Thanks,
Communication Type:
Discontinuation Notice
V#10605
-
06/5/2025
Title: Medline V#10605
Details:
Medline Enzymatic Reformation Update-Concordance
Customer Listing for the past 6 month is attached here.
Original Notification from Medline is attached here.
Dear Distributor Partners,
We’d like to inform you that several of our detergent item numbers have recently undergone reformulation.
These updates were made to incorporate safer, more sustainable ingredients while enhancing overall product efficacy. The only noticeable differences your customers may observe are slight changes in color and scent, which are a result of the more natural formulation.
For your reference, a detailed memo is attached in case customers have any questions.
Detergent Reformulation Summary
- Prop 65 warnings will be removed from packaging.
- GHS hazard classifications are now reduced, reflecting safer ingredients.
- Slight changes in color and scent may be noticeable due to more natural components.
- All reformulated products are tested and certified to:
- ASTM D7225 (for automatic washers, ultrasonic cleaners, and manual cleaning)
- ASTM 8179 standards
- Improved cleaning efficacy across all applications.
Affected Product Lines & Item Numbers
- Single Enzymatic: MDS88000B91, MDS88B9105, MDS88B9115
- Dual Enzymatic: MDS88000B9, MDS8800B95, MDS8800B15
- Multi Enzymatic: MDS88128ME, MDS8805ME, MDS8815ME
Foam Presoak Spray: MDS8800FOAM
Communication Type:
Product Changes
V#10605
-
06/5/2025
Title: B Braun V#10508
Details:
B. Braun Clearly Safe Awareness Campaign -- launches today!!
We want to make you aware of our upcoming B. Braun Clearly Safe awareness Campaign (www.bbraunusa.com/clearlysafe).
For decades, scientists have recognized the risks of exposure to Di-(2-ethylhexyl) phthalate (DEHP). DEHP is an endocrine-disrupting chemical associated with serious adverse health effects, including cancers and reproductive disorders.1 DEHP can leach into IV fluids, potentially putting patients at risk. In September 2024, the California Toxic-Free Medical Devices Act (AB 2300) was signed into law to prohibit the manufacture, sale or distribution of IV solution containers and IV tubing made with DEHP.
The reason our IV bags aren’t made with DEHP is clear. Over 40 years ago, B. Braun recognized the environmental and patient risks of toxic chemicals like DEHP. Today, B. Braun is the only supplier offering a full line of IV drugs, IV and nutrition solutions, and irrigation containers plus a robust line of IV sets and connectors not made with DEHP.
We encourage you to act:
- Ask the FDA to update its guidance: Sign the Petition
- Ask for an educational presentation on DEHP: Send a Request
Throughout our campaign, we will be raising awareness of this important topic. Please be on the lookout for our LinkedIn post, repost/share as you wish. As always, if you have questions, please contact myself or Sarah.
Thank you!
Julie Bauer RN BSN
Associate Director, Channel Marketing, Distribution
Email | julie.bauer@bbraunusa.com
Mobile | 610-714-2615
B|BRAUN – Sharing Expertise
Communication Type:
Updated Supplier Information
V#10605
-
06/5/2025
Title: Medegen V#10423
Details:
6.4.25 Medegen notification_disco mfg#1032-07
Concordance Team,
Medegen V#10423 has discontinued mfg #1302-07 and does not have a replacement product.
Communication Type:
Discontinuation Notice
V#10423
-
06/5/2025
Title: Baxter V#10797
Details:
Concordance Team,Baxter V#10797 announced that the following IV sets have been removed from allocation, and updates have been made in our system accordingly. See the customer listing attached.

Communication Type:
Allocation Notification/Update
V#10797
-
06/4/2025
Title: Angelini V311062
Details:
Please find attached a letter from our Quality Department on shipping recommendations for our Alcavis 50 and ExSept Plus products.
Thank You,
Scott Livingston
BUSINESS DEVELOPMENT MANAGER
Angelini Pharma Inc.
230 Peachtree Street NW, Suite 1250, Atlanta, GA 30303
M +1-847-651-1068
Communication Type:
Recall Notification
V#11062
-
06/4/2025
Title: Aspen Surgical V#10234
Details:
Aspen Surgical/Bovie Medical Surgical Lighting via NDC.
We have provided NDC with periodic updates on Aspen’s strategic decision to discontinue supplying Medical Illuminations Lighting and now we will be provide Simeon Medical Lighting.
Attached is Concordance’s pricing on Simeon Medical Light, if they wish to purchase directly. The option to purchase via NDC is still available. Additional details on MI to Simeon Crosses and Simeon Medical information is attached.
Please let me know if you have any questions.
Tyler Farmer
CHANNEL PARTNER & OEM SALES MANAGER
tyler.farmer@aspensurgical.com
PHONE / +1 616.204.7195
CUSTOMER SERVICE / +1 888.364.7004
FAX / +1 888.364.5381
6945 Southbelt Dr. SE, Caledonia, Michigan 49316 USA
aspensurgical.com
Aspen™ Bard-Parker® Bookwalter® Bovie® Olsen® Precept® Symmetry®
Communication Type:
Discontinuation Notice
V#10234
-
06/4/2025
Title: Vygon V#10242 - Sales Map/Roster
Details:
Sales Territory Map - 6.2.2025
Concordance Team,
Please see the attached sales map from Vygon USA.
with any further questions, please reach out to Jeff Shook.
Kind Regards
Communication Type:
Sales Roster
10242
-
06/3/2025
Title: Roche V#10603
Details:
Upcoming Roche V# 10603 holiday order fullfillment schedule
Concordance Team,
FROM ROCHE DIAGNOSTICS: "Please see the calendar below for Roche order fulfillment information, given the Juneteenth and July 4th Roche holidays."

Communication Type:
Holiday Schedule
V#10603
-
06/2/2025
Title: J&J V#10196
Details:
J&J Surgifoam & Gut update email
Concordance Team,
The following items have been removed from allocation and a customer listing is attached.
CHS#735563 mfg#1973
CHS#168267 mfg#1975
Communication Type:
Allocation Notification/Update
V#10196
-
06/1/2025
Title: Nestle V#10183
Details:
Attached is a product update from Nestle Health Science. Boost side by Side letter Please contact your distributor manager with questions.
Take care,
Amy Sauber, RDN, LDN
Manager, Sales Support Team
Nestlé Health Science U.S.
CONFIDENTIALITY NOTIFICATION: This email message and any attached documents are intended only for the use of the intended recipient(s) and may contain information that is confidential. If you have received this email in error, please notify the above sender and delete it from your computer.
Communication Type:
Product Changes
V#10183
Communication Type:
Product Flyer
V#10183
-
05/30/2025
Title: Health-O-Meter V#10075
Details:
ADA Compliant Scale flyer
Below is an announcement about our ADA Compliant scales to send out to the Sales Team for the upcoming month along with the flyer attached. Please let us know if you have any questions or if we can provide anything further.
Health o meter® Professional Stand-On Scales Certified as ADA Compliant
Health o meter® Professional Scales is proud to announce that our top-selling platform scales, the 1100KL and 2101KL, are now officially certified as ADA compliant. These scales meet the stand-on scale requirements listed in the US Access Boards new MDE standards.
These requirements include:
- Slip resistant platform
- Handrail supports on both sides of platform
- 34" - 38" max. handrail height from platform
Health o meter® Professional Scales offers the only ADA Compliant scales in the market that are independently certified by a third party, supporting healthcare facilities in providing accessible, compliant, and inclusive care.
See the attached flyer to learn more about our ADA Compliant products. Please reach out to Brett Lucido, Director of National Accounts, blucido@homscales.com, (724) 799-4233 if you have any questions or would like more information.
thanks,
Kelly Virzi
Sales & Marketing Specialist
- 1-708-377-0549
- kvirzi@homscales.com
9500 West 55th Street, McCook, IL 60525-7110 USA
Pelstar, LLC
Health o meter® Professional Scales | Follow Us
weigheasier®
Communication Type:
Product Flyer
V#10075
Communication Type:
Product Update
V#10075
-
05/30/2025
Title: Medela V#10262
Details:
See attached document with regards to the product discontinuation and product replacement.
Product Being Discontinued
101041360 Pump In Style MaxFlow
101041359 Pump In Style MaxFlow (WIC)
Product Replacement
101047090 Pump In Style Pro
101047093 Pump In Style Pro (WIC)
Thank you.
John Solet
Sr. Strategic Account Manager
M:404-561-9387
Communication Type:
Discontinuation Notice
V#10262
-
05/30/2025
Title: Midmark V#10987
Details:
Please see attached notification and list of new and discontinued products.
Thank you.
Designing better care.®
Midmark Corporate Accounts Department
T: 1.800.MIDMARK
midmark.com
CONFIDENTIALITY NOTICE: This message, including any attachments, contains confidential information intended for a specific individual and purpose. If you are not the intended recipient, you should delete this message and any disclosure, copying, or distribution of this message, or the taking of any action based on it, by you is strictly prohibited.
Communication Type:
Discontinuation Notice
V#10987
-
05/30/2025
Title: Getinge V#10043
Details:
Healthmark/Getinge will be discontinuing the Getinge Clean (GetClean)- Cleaning chemistries May 31, 2025. Healthmark/Getinge has introduced a new chemistry line called Xen. Please reach out if you would like more information on Xen.
Regards,
Samantha Blay
Healthmark Industries Co.
Contract Dept. Supervisor18600 Malyn
Fraser, MI 48026This e-mail and any files transmitted with it are confidential and intended solely for the use of the addressee(s). It may contain confidential, proprietary and/or legally privileged information. If
Communication Type:
Discontinuation Notice
V#10043
-
05/29/2025
Title: CryoConceptsV#12777
Details:
hope you have had a terrific week! As a follow up to the CryoConcepts product review on Tuesday morning, I am attaching a few documents. All of these should also be available via HubSpot. THANK YOU AGAIN FOR YOUR TIME AND ATTENTION – LET’S GROW BUSINESS TOGETHER!
- Concordance Branded Histofreezer FLEX Brochure: remember that the sweet spots include: non-derm primary care – pediatrics, family practice, internal medicine, OBGYN, urgent care, and podiatry.
- NEW EPA Rules Brochure: the new EPA Regulation is in effect right now (and as of 1/1/25)! If you have accounts that are currently using Verruca Freeze, CryoDose, or any private label Verruca Freeze, these accounts are now effected and will benefit from switching over to Histofreezer FLEX or any one of other products.
- Concordance Q2 Incentive: EXCLUSIVE TO CONCORDANCE. Earn additional money for every CryoConcepts product sold between 4/1 – 6/30!
- Histofreezer FLEX GREEN & SUSTAINABLE Brochure – remember, we are the only cryo product that your customers can return at no charge for recycle and re-use. This also helps with system and practice sustainability initiatives.
- NEW: HISTOFREEZER FLEX single units available for sale & local product stocking across multiple distribution centers!
Also, remember that any primary care account (non-dermatology) that you cover that is currently using liquid nitrogen is also a perfect target! Please contact me or David with any and all opportunities, big or small!!!
Have a wonderful holiday weekend!
Doug
Communication Type:
Product Flyer
V#12777
-
05/28/2025
Title: Coloplast V#10581
Details:
USCC_Freedom Clear Discontinuation_Letter_PM-38147_F
USCC_Freedom Clear Discontinuation_Letter_PM-38147_F
PM-35881 Freedom Clear discontinuation conversion list_HCP_F
PM-35885 Freedom Clear discontinuation Conveen Optima HCP fact sheet_F
Freedom Estimated Runout Excel 05.06.2025
Vendor Item List Sales History 11.28.24-5.28.25
Concordance Team,
From Coloplast V#10581: "Coloplast will be discontinuing the Freedom Clear line of male external catheters. This transition will happen by sku between June and into next year. We will convert users over to our Conveen Optima line of external catheters. The two items to first focus on are: 5490 Freedom Clear 35mm 5290 Freedom Clear 28mm
Attached is an EXCEL sheet which has the estimated runout date by item. This also has the Conveen Optima cross which you can start to transition customers to. Please feel free to start transitioning all of these items now, if that is more convenient for you. Also included are a few resources which will be helpful for you in this transition."
Communication Type:
Discontinuation Notice
V#10581
-
05/28/2025
Title: Molnlycke V#10008
Details:
Hibiclens 32oz & Gal Supply Update Notification
Vendor Item List Sales History 10.17.24-4.17.25
Concordance Team,
Molnlycke V#10008 sent an update regarding the discontinuation of two items previously announced. Instead of July 1, the below two items are discontinued effective immediately. They have exhausted their inventory sooner than estimated. An updated notification letter is attached.
- 57532 HibiClens 32oz bottles (C#219485)
- 57591 HibiClens 1-gallon bottles (C#219469)
Communication Type:
Discontinuation Notice
V#10008
-
05/27/2025
Title: Cardinal V#10801
Details:
Premier notice on Tariff prices
Tariff-related Price Increases
For several weeks, Cardinal Health has been in discussions with Premier regarding the need to make temporary tariff-related price adjustments on contracted products. The proposed tariff increases were only for the following categories:
- Disposable Non-sterile Protective Apparel
- Sterile Packs and Gowns
- Suction Canisters, Yankauers and Tubing
Premier’s member committee reviewed the tariff-related increase requests provided by Cardinal Health and voted to deny the request for increases. Cardinal Health has rescinded the proposed temporary price increase requests for these categories and will maintain Premier contracts for these products.
While successfully avoiding contract terminations and double-digit increases in three categories, Cardinal Health continues to push forward price increases that are contractually allowed.
- The table below outlines the initial proposed tariff increases, the amounts permitted per the contract terms, the increases Cardinal Health is likely implementing and the mitigation percent thus far.
- Cardinal Health has indicated they are revising member impact sheets they previously shared directly with members and will be reviewing those with select organizations in the next few days.
- Based on results below, Cardinal Health will work with distributors on revised price file loads as necessary.
Our next Member Committee Tariff Review meeting is scheduled for May 29. We will update the member account management teams as necessary after that meeting. Thank you for your continued patience and support as we work through these challenging events.
Communication Type:
Tarriff
V#10801
-
05/27/2025
Title: Baxter V#10797
Details: Baxter allocation update for 5.27.25
Concordance Team,
Baxter announced two items are being removed from allocation effective today, 5.27.25. The below items were removed from allocation in our system this morning, and a customer listing is attached for those using that can be notified.
CHS#657171 mfg#2F7112
CHS#637397 mfg#2F7122
Communication Type:
Allocation Notification/Update
V#10797
-
05/23/2025
Title: Hemocue V#10452
Details:
New pricing effective July 7th- Please see below.
Dear HemoCue Partner:
Due to recently imposed tariffs on products imported to the US, we are reaching out to
inform you of an upcoming surcharge for HemoCue America’s products.
We have carefully considered how to handle the increased costs of tariffs and have
concluded that, to help support our security of supply commitments to you, it is
necessary for us to implement an unavoidable tariff surcharge in relation to the
shipment of our products based on current tariff rates for their country of origin. All
surcharges are effective July 7, 2025, which is 45 days from the date of this letter.
Please see attachment for new pricing by product.
We recognize that circumstances are evolving, and we will continue to monitor the
trade situation closely. If tariffs increase or decrease in the future, we will review
surcharges accordingly and adjust as appropriate. The timing of any potential decrease
will be subject to inventory levels on-hand at that time by HemoCue America. In other
words, if tariffs decrease in the future, current inventory – which would have been
subject to tariffs – will need to be depleted before any tariff surcharge decrease. No
credits will be given for previous purchases under the tariff surcharge, should a tariff be
reduced or eliminated.
While we appreciate that the introduction of a surcharge may pose challenges for you,
please be assured that the decision was made after exploring a range of options to
manage the increase in our costs without compromising our ability to serve you in the
way that you expect from us.
We greatly value our business with you and believe in transparency and open
communication. We will continue to monitor the trade situation and increased costs
closely and will communicate as soon as possible any further changes that may become
necessary.
If you have any questions, please reach out to Brett Basil, Senior Distribution Sales
Manager at 312-636-9673 or brett.a.basil@hemocue.com.
Thank you for your continued partnership with HemoCue.
Best regards,
Jack Van’t Groenewout
Senior Director, Global SalesCommunication Type:
Tarriff
V#10452
-
05/23/2025
Title: BD V#10125
Details:
UPDATED Allocation tool for May 2025
- Several items have been added.
- Includes a listing of the sales rep you need to reach out to request a change in allocation.
Please reach out to Heather Burkett if you have any questions. Thanks,
Communication Type:
Allocation Notification/Update
V#10125
-
05/22/2025
Title: V#12008
Details:
Concordance Team,
Please be advised that Geri-Care will be closed from June 2nd through June 3rd for the Shavuot holiday. They will be back in office on Wednesday, June 4th.
Communication Type:
Holiday Schedule
V#12008
-
05/22/2025
Title: Abbott V#10575
Details:
We are sharing with all of our distributor partners the attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File 5.22.25 Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Chad Truini
Sr. Business Analyst
Commercial Operations
Abbott Nutrition
3300 Stelzer Road
Columbus, OH 43219
O:
+1 614-624-0239
This communication may contain information that is proprietary, confidential, or exempt from disclosure. If you are not the intended recipient, please note that any other dissemination, distribution, use or copying of this communication is strictly prohibited. Anyone who receives this message in error should notify the sender immediately by telephone or by return e-mail and delete it from their computer.
Communication Type:
Product Inventory Update
V#10575
-
05/22/2025
Title: Abbott V#10575
Details:
Below is the grid we sent with the attached letter via email on October 30th, 2024. Please add your replacement item information in the yellow shaded column.
Distributor Acquisition Cost (3)
April 2025 - June 30, 2025
Distributor Acquisition Cost (3)
Effective July 1, 2025
Item
Current Abbott Item #
Current Concordance Item #
NEW Abbott Item #
NEW Concordance item #
New Item UPC (Case)
New Item UPC (Unit)
New Item NDC Format Code (Unit) (1)
Estimated Launch Timing (2)
1 - 124 Case Price
125+ Case Price
1 – 124 Case Price
125+ Case Price
EleCare Jr Unflavored 14.1-oz can
55253
181217
68625
413493
70074686257
70074686288
70074-0686-28
April-25
$249.64
$239.64
$268.66
$258.66
EleCare Jr Vanilla 14.1-oz can
56585
185013
68630
413502
70074686301
70074686318
70074-0686-31
April-25
$249.64
$239.64
$268.66
$258.66
(1)NDC-format codes: NDC Format codes are product codes adjusted according to standard industry practice to meet the format requirements of pharmacy and health insurance computer systems. Abbott Nutrition does not represent these products to be drugs, nor their codes to be NDC codes. If used for third-party billing purposes, each healthcare provider or contracted vendor is ultimately responsible for the accuracy and appropriateness of codes billed for individual patients and services.
(2) Estimated launch timing is subject to change.
(3) 125 Cs Price / Case: All Abbott products have bracketed pricing based on the quantities ordered which is the price that is reflected on distributor's invoices from Abbott. For Contract Pricing (TN) the 125-case contract price is your Cost-Basis. The Cost-Basis price brackets are the cells outlined in red.
We appreciate anything you can do to expedite the set-up of these replacement items in your system. Please reach out to me or Joe Corey with any questions.
Thank you,
Chad Truini
Sr. Business Analyst
Commercial Operations
Abbott Nutrition
3300 Stelzer Road
Columbus, OH 43219
O:
+1 614-624-0239
Communication Type:
Discontinuation Notice
V#10575
-
05/21/2025
Title: Medline V#10605
Details:
Discontinuation Notice
Please see below notification from V#10605, Medline. C#954248 has been replaced by C#414505. There are additional subs below as well.
Attached is a listing of customers who have purchased in the past 6 months. Please reach out if you have any questions. Thanks,
DYNJ04058 has been discontinued and replaced by DYNJ04058A.
Demand, forecasting and pricing have all been updated to allow you to order product immediately; however, we are working with our supplier to regain a healthy level of inventory. In the meantime, we have some other options that may suit your needs.
The best alternative options at this time are below:
* DYNJ04058Z – same item, different UOM
* DYNJ04062 – same item in a kit with gauze included
* DYNJ04059 – plastic handle (we have more inventory available here to break down to meet LUM demand

Communication Type:
Discontinuation Notice
V#10605
-
05/21/2025
Title: ICU V#10917
Details:
Please see attached notification from ICU, V#10917. ICU is recommending that end users switch from C#365588 and C#365541 to the new codes (6381, 6380, 6390 and 6391) this week. Attached is a listing of customers who have purchased C#365548 and C#365541 in the past 6 months.
Please reach out to Heather Burkett, if you have any questions.
Communication Type:
New Product Launch
V#10917
Communication Type:
Product Inventory Update
V#10917
-
05/21/2025
Title: BD V#10125
Details:
Please see attached notification from BD as well as cross reference guide.
All of the original items listed in the letter are have been either inactive or temporarily unavailable for some time so there isn’t a listing of customer sales to run. Please let Heather Burkett know if you have any questions.
Communication Type:
Product Inventory Update
V#10125
Communication Type:
Product Update
V#10125
-
05/21/2025
Title: Invacare V#10748
Details:
Offices Closed In Observance of Shavuot:

Communication Type:
Holiday Schedule
V# 10748
-
05/21/2025
Title: Microcare V#10201
Details:
Tariff letter
May 16, 2025
Dear Valued Customer:
Thank you for your continued trust and partnership with MicroCare.
As follow up to our update letter dated April 17, 2025, we want to inform you that despite the recently announced 90 day freeze on tariffs between the U.S. and China, we are experiencing tariff-related cost increases from many of our suppliers. As a result, we will be adjusting the prices of several MicroCare products effective June 2, 2025.
To support your planning efforts, we will honor current pricing for any orders scheduled to ship on or before May 30, 2025.
These pricing adjustments reflect the current environment and take into account the most recent tariff developments. We will continue to monitor the situation and should tariffs be reduced or eliminated, we will promptly update our pricing accordingly.
At MicroCare, we are committed to domestic manufacturing and supporting our customers through supply chain challenges. Our team is available to assist with quotes, product availability, and any questions you may have.
We appreciate your understanding and continued support. Please don’t hesitate to contact your MicroCare representative for assistance.
Sincerely,
Thomas Tattersall
Chief Executive Officer
Communication Type:
Tarriff
V#10201
-
05/20/2025
Title: Medtronic V#10255
Details:
Medtronic Med Surg Shiley Product Notification May2025
Medtronic Med Surg Shiley Product Notificaton May25
Vendor Item List Sales History 11.19.25-5.18.25
Concordance Team,
Please see the attached discontinuation notice from Medtronic.
- The listed products are being discontinued on 06/30/2025 or until their inventory is depleted.
- The attached file named VAI Item_cross ref list indicates the only items set up in our system.
- A 6-month usage report is attached.
Communication Type:
Discontinuation Notice
V#10255
-
05/20/2025
Title: Medtronic V#10255
Details:
Medtronic Foley Catheter Substitution Letter - 05-2025
Medtronic ACM Mon-A-Therm Foley Cath Discontinuation Notification 5-15-25
Vendor Item List Sales History 11.18.24-5.18.25
Concordance Team,
Please see the attached discontinuation notice from Medtronic V#10255 along with the replacement items. As noted, this is effective 5/15/25, and all open orders for the discontinued codes, if any, have been canceled by them.
A 6-month usage report is attached. Reach out to our Item Maintenance team if there is customer demand for the suggested replacement items, and they can set them up in the system.
Communication Type:
Discontinuation Notice
V#10255
-
05/19/2025
Title: Ansell V#11003
Details:
May 16, 2025 TARIFF
Dear Valued Partner,
Effective May 14, the Trump Administration lowered the China “reciprocal” tariff rate to 10%, aligning it with the rate implemented for other countries on April 5. This 10% is in addition to the 20% tariff imposed on March 4, bringing the total tariff on Chinese goods to 30%.
In light of this change, we will defer the previously announced June 1 price increase for China manufactured products. Please note that all other previously announced pricing actions remain in effect and unchanged; this includes the June 1 increase on styles manufactured outside of China, and the initial May 1 increase on styles manufactured in China.
Attached please find a list of all Ansell styles made in China. To ensure timely order processing, please continue to use the May 1 pricing for these styles only. While the China tariff reduction is a welcome development, we recognize that it necessitates work on your end, and that as price books reflecting the previously announced June 1 increase have already been distributed, you may have already loaded pricing. We’re committed to making this update as smooth as possible, and encourage you to reach out at any time with questions or for support with placing your orders.The deferred price increase for China manufactured products will now take effect July 1; pricing for end-user contracts will also be updated accordingly, effective July 1. The exact increase amount will be shared along with revised price books, which we expect to begin sending early-mid June. In the meantime, we would like to assure you that Ansell has maintained and will continue to deliver consistent and reliable supply across our portfolio, including styles impacted by the elevated tariffs on China-manufactured products. Despite the added cost pressures, our priority remains ensuring uninterrupted supply to support you and your customers’ safety needs. We appreciate your patience as we navigate this dynamic environment.
Thank you again for your continued trust and partnership.
Kind Regards,
Sean Sweeney
Chief Commercial Officer AmericasCommunication Type:
Tarriff
V#11003
-
05/19/2025
Title: Cardinal V#10801
Details:
60 ML Discontinued Letter 5 MO customer usage
We have also made the decision to discontinue the following syringes due to low demand. We have limited inventory available and suggest referencing the replacement product for your next order placement. 1181200777 will be available in December 2024
Communication Type:
Discontinuation Notice
V#10801
-
05/19/2025
Title: Angelini Pharma V#11062
Details:
As a valued Angelini customer, we wanted to update you on our Alcavis Bleach Wipe availability.
Please find attached a letter outlining the situation and let us know if you have any questions.
Have a great weekend,
Scott Livingston
BUSINESS DEVELOPMENT MANAGER
Angelini Pharma Inc.
230 Peachtree Street NW, Suite 1250, Atlanta, GA 30303
M +1-847-651-1068
Communication Type:
Backorder Notification/Update/Reports
V#11062
-
05/19/2025
Title: PDI V#10497
Details:
PDI WIPES LETTER and YTD USAGE
- Nice ’n Clean Baby Wipes have been discontinued. There is no price change for the new PDI-branded replacements:
- Discontinued M233XT: Nice ’n Clean Unscented Baby Wipe 80ct → Replaced with M41580: PDI Unscented Baby Wipe 80ct
- Discontinued Q70040: Nice ’n Clean Unscented Baby Wipe 40ct → Replaced with A62640: PDI Unscented Baby Wipe 40ct
Communication Type:
Discontinuation Notice
V#10497
- Nice ’n Clean Baby Wipes have been discontinued. There is no price change for the new PDI-branded replacements:
-
05/18/2025
Title: Kimberly Clark V#10765
Details:
41482 - Kimberly Clark - allocation
Vendor Item List Sales History 11.16.25-5.15.25
Concordance Team,
The attached allocation letter for mfg#41482 C#172246 from Kimberly Clark was recently received. Based on our historical usage, Kimberly Clark how stated Concordance will have a 20-case allocation per month. One case has been allocated in our system to TIFF, SHEL, ZOO, and WCST and the rest to CRIV. The customer usage file is attached.
Communication Type:
Discontinuation Notice
V#10765
-
05/18/2025
Title: Baxter V#10797
Details:
Inlet Allocation_Customer Letter May 2025_vF
Concordance Team,
In reference to the attached Baxter letter, the following items are on allocation for the amounts shown. A customer listing is attached, indicating two customers to be notified about just three of these items.
CHS#112511 H938173 75%
CHS#112510 H938174 50%
CHS#110970 H938175 75%
CHS#113796 H938176 75%
Communication Type:
Allocation Notification/Update
V#10797
-
05/16/2025
Title: Avanos V#11967
Details:
Important Update – Product Code Change for ESENTEC™ Conventional Cannulae Portfolio
Please see letter from Avanos, V#11967 regarding discontinuation. Attached is a listing of all end users for the past 6 months as well as a conversion file with replacement items. Please note, the replacement items are not set up in VAI. Please reach out if you have any questions. Thanks,
Please reach out to Heather Burkett if you have any questions.
Dear Valued Partner,
We are writing to inform you of an important update to the cannula within the Avanos RFA Solutions ESENTEC™ line. As a part of our commitment to Supply Chain resiliency, we are removing the platinum band from our conventional cannulae due to long term raw material availability risks. This helps ensure we can more confidently provide consistent production and supply.
This change does shift the previous PMF code make-up to a new PMC version and a table with the updated product codes, showing both the current PMF version and the new PMC equivalents, can be found here. Please note that this update does not affect product specifications, quality, or performance.
The existing inventory of the PMF version will be phased out starting in April 2025. Once the current inventory is depleted, we will no longer replenish stock of the PMF product and will be shifting to the stock available in our PMC code versions.
To ensure a smooth transition, we recommend adding the PMC product codes to your internal systems to ensure you can order the new version once the PMF stock has depleted. Our Customer Service team is available at 1-844-4AVANOS to assist with any questions or support you may need.
Thank you for your attention to this update and for your continued partnership.
Communication Type:
Discontinuation Notice
V#11967
-
05/16/2025
Title: B Braun V#10508
Details:
May Allocation File
Please see attached May Allocation Files and Sales Rosters.
On the file, “Current Allocation – May 2025 5_15”
- Column F (ARF- allocation request form) is whether B Braun is allowing additional allocation increases
- Column E is what percentage of allocation they will be able to fill for May
I have also attached a current sales roster for all of B Braun in case you need to reach out for additional allocation increases.
Let me know if you have any questions. Thanks,
Communication Type:
Allocation Notification/Update
-
05/15/2025
Title: Abbott V#10575
Details:
We are sharing with all of our distributor partners the attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File 5.15.25 Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Chad Truini
Sr. Business Analyst
Commercial Operations
Abbott Nutrition
3300 Stelzer Road
Columbus, OH 43219
O:
+1 614-624-0239
Communication Type:
Product Inventory Update
V#10575
-
05/14/2025
Title: Baxter V#10797
Details:
Baxter has removed nearly all of the IV fluid allocations produced in their North Cove, NC, manufacturing facility, and our system has been updated accordingly. A cross reference to Concordance item numbers and customer listing is attached for those needing notified.
From the 4/29/25 allocation letter: Below is summary of notable advancements related to North Cove that has helped us achieve these key milestones and will help support your facility’s return to normal practices:
- All North Cove manufacturing lines are producing at or above pre-hurricane levels of output
- Baxter is carrying over 120% of pre-hurricane inventory levels for North Cove products at our distribution centers
- Baxter has 8 weeks of inventory on top 11 Product Codes that represent 85% of IV Solutions usage within U.S. facilities, at our distribution centers
- In March, we shipped over 2 million units of product made in North Cove to support our distributor partners’ replenishment goals
Communication Type:
Allocation Notification/Update
V#10797
-
05/14/2025
Title: Solventum V#10296
Details:
188 -EN_MedSurg-Reston Discontinuation-NR - FINAL- April 2025
Vendor Item List Sales History 11.14.25-5.13.25
Concordance Team,
V#10296-Solventum's med surge business is discontinuing the four items listed in the attached notice. Solventum said the target discontinuation date is July 2025.
 They will continue to sell these products to existing customers until the inventory is depleted.
They will continue to sell these products to existing customers until the inventory is depleted.- Mfg #1560M C#18412
- Mfg #1561H C#181420
- Mfg #1563L C#181438
- Mfg #2851H---N/A. This is not set up.
Communication Type:
Discontinuation Notice
V#10296
-
05/14/2025
Title: PDC V#10751
Details:
PDC Tape Shortage Notice
Please see attached for notice from PDC on tape shortage they are experiencing. Also attached is a listing of tapes purchased in the past 12 months as well as a customer listing for end users who have bought the tapes purchased in the past 6 months.
If you have any questions please reach out to Heather Burkett. Thanks,
Communication Type:
Backorder Notification/Update/Reports
V#10751
-
05/14/2025
Title: Medline V#10605
Details:
Supply Constraints on Knit Pants
Please see letter below from Medline and please reach out to the supplier if you need a sub. Attached is a listing of customers who have purchased in the past 6 months.
A select subset of knit pants (below) will experience spotty backorders due to spikes in demand and extended vendor lead times to replenish. Details on SKUs impacted and timing in the table below.

We do not have sufficient supply of substitute options, so not all scenarios will be eligible for a substitute. We will manage the use of substitutes on an individual request level.
Communication Type:
Backorder Notification/Update/Reports
V#10605
-
05/14/2025
Title: BD V#10125
Details:
Holiday Schedule
Dear Channel Partner,
To help effectively plan your order fulfillment needs, enclosed is a copy of BD’s 2025 Holiday Schedule.
Kindly note the following:
Memorial Day (Monday, 5/26):
- BD fully closed (no Customer Care coverage will be available)
- No deliveries
- Milk Runs from Sparks, MD normally scheduled to ship Monday, 5/26 will depart Saturday, 5/24 and deliver on their normal delivery day
- Milk Run Shipments from Sparks that would normally deliver Monday, 5/26 will be delivered Tuesday, 5/27
- No temperature sensitive Parcels will be shipped on 5/23 or 5/26
Thank You,
BD Supply Chain Relations
Communication Type:
Holiday Schedule
V#10125
-
05/14/2025
Title: Pfizer V#12114
Details:
Introducing
Doxorubicin Hydrochloride Injection per Single-dose Vial for Novaplus® Products10 mg/5mL (NDC 0069-5629-05)
20 mg/10mL (NDC 0069-4031-12)May 12, 2025
Dear Valued Customer,
Great News! Pfizer Hospital is pleased to announce the availability of Doxorubicin Hydrochloride Injection 10 mg (5mL), and 20 mg (10mL) per Single-dose Vial for Novaplus® Products.Inventory is available and ready for shipment to your distribution centers effective immediately.
Product Description
Unit of Sale NDC
Unit of Use NDC
List Price
Doxorubicin Hydrochloride Injection 10 mg/5 mL
per Single-dose Vial for Novaplus®
(1 Carton of 1 Vial)0069-5629-05
0069-5629-05
$10.15
Doxorubicin Hydrochloride Injection 20 mg/10 mL
per Single-dose Vial for Novaplus®
(1 Carton of 1 Vial)0069-4031-12
0069-4031-12
$20.30
Please click the links below to review important product information.
Click here to view USPI for Novaplus® Doxorubicin Hydrochloride Injection
Click here to view SDS
Click here to view Novaplus® Doxorubicin Hydrochloride Injection 10 mg (5mL) HDA Product Information Form
Click here to view Novaplus® Doxorubicin Hydrochloride Injection 20 mg (10mL) HDA Product Information Form
Click here to view Novaplus® Doxorubicin Hydrochloride Injection 10 mg (5mL) Product Images
Click here to view Novaplus® Doxorubicin Hydrochloride Injection 20 mg (10mL) Product ImagesShould you require additional information, please contact your Pfizer Hospital Trade National Account Director or Customer Service at 1-844-646-4398.
Sincerely,
Pfizer HospitalCommunication Type:
New Product Launch
V#12114
-
05/14/2025
Title: Pfizer V#12114
Details:
Introducing
Sterile Water for Injection, USP 10mL per Single-dose Fliptop Vial for Novaplus®NDC 0409-0024-10
May 12, 2025
Dear Valued Customer,
Great News! Pfizer Hospital is pleased to announce the availability of Sterile Water for Injection, USP 10mL per Single-dose Fliptop Vial for Novaplus®.Inventory is available and ready for shipment to your distribution centers effective immediately.
Product Description
Unit of Sale NDC
Unit of Use NDC
List Price
Sterile Water for Injection, USP 10mL per Single- dose Fliptop Vial for NovaPlus®
(1 Tray of 25 Vials)0409-0024-10
0409-0024-01
$28.55
Please click the links below to review important product information.
Click here to view USPI for NovaPlus® Sterile Water for Injection
Click here to view SDS
Click here to view NovaPlus® Sterile Water for Injection HDA Product Information Form
Click here to view NovaPlus® Sterile Water for Injection Product ImagesShould you require additional information, please contact your Pfizer Hospital Trade National Account Director or Customer Service at 1-844-646-4398.
Sincerely,
Pfizer HospitalCommunication Type:
New Product Launch
V#12114
-
05/14/2025
Title: Pfizer V#12114
Details:
Introducing
Cytarabine Injection 2 g/20mL (100 mg/mL)
per Single Dose Fliptop Vial for HealthTrustNDC 61703-155-01
May 12, 2025
Dear Valued Customer,
Great News! Pfizer Hospital is pleased to announce the availability of Cytarabine Injection 2 g/20mL (100 mg/mL) per Single Dose Fliptop Vial for HealthTrust.Inventory is available and ready for shipment to your distribution centers effective immediately.
Product Description
Unit of Sale NDC
Unit of Use NDC
List Price
Cytarabine Injection
2 g/20mL (100mg/mL) per Single Dose Fliptop Vial for HealthTrust
(1 Carton of 1 Vial)61703-155-01
61703-155-01
$20.32
Please click the links below to review important product information.
Click here to view USPI for Cytarabine
Click here to view SDS
Click here to view Cytarabine Injection 2 g/20mL for HealthTrust HDA Product Information Form
Click here to view Cytarabine Injection 2 g/20mL for HealthTrust Product ImagesShould you require additional information, please contact your Pfizer Hospital Trade National Account Director or Customer Service at 1-844-646-4398.
Communication Type:
New Product Launch
V#12114
-
05/14/2025
Title: Pfizer V#12114
Details:
Pfizer Hospital Announces a NDC Change for
Epinephrine Injection, USP
1 mg/10mL (0.1 mg/mL) per Abboject® Single-Dose SyringeNDC 0409-4933-10
May 12, 2025
Dear Valued Customer,
Please click the links below to review important information regarding Epinephrine Injection, USP 1 mg/10mL (0.1 mg/mL) per Abboject® Single-Dose Syringe NDC change.
Click here to view the Customer Letter
Click here to view the Epinephrine Injection, USP 1 mg/10mL current images and new imagesPlease click the links below to review product information for the new
Epinephrine Injection, USP 1 mg/10mL NDC.Click here to view USPI
Click here to view SDS
Click here to view Epinephrine Injection, USP 1 mg/10mL HDA Product Information Form
Click here to view Epinephrine Injection, USP 1 mg/10mL Product ImagesShould you require additional information, please contact your Pfizer Hospital Trade National Account Director or Customer Service at 1-844-646-4398.
Sincerely,
Pfizer HospitalThe information provided is accurate at the time of this communication, and is subject to change without notice. Pfizer is not responsible for systems and databases external to Pfizer that may use this information in its current form or as superseded by future changes.
Please note that Average Wholesale Price (AWP) is set independently by compendia without input from Pfizer Inc. Pfizer plays no role in setting AWP and defers to compendia with respect to how AWP is determined.
Communication Type:
Product Changes
V#12114
-
05/14/2025
Title: Pfizer V#12114
Details:
Pfizer Hospital Announces a NDC and Pack Size Change for
Marcaine™ 0.75% (bupivacaine HCI Injection, USP)
225 mg/30mL (7.5 mg/mL) per Single-Dose VialNDC 0409-2253-25
May 12, 2025
Dear Valued Customer,
Please click the links below to review important information regarding Marcaine™ 0.75% (bupivacaine HCI Injection, USP) 225 mg/30mL (7.5 mg/mL) per Single-Dose Vial NDC and Pack Size change.
Click here to view the Customer Letter
Click here to view the Marcaine™ 0.75% (bupivacaine HCI Injection, USP) 225 mg/30mL current images and new imagesPlease click the links below to review product information for the new
Marcaine™ 0.75% (bupivacaine HCI Injection, USP) 225 mg/30mL NDC.Click here to view USPI for Marcaine™
Click here to view SDS
Click here to view Marcaine™ 0.75% (bupivacaine HCI Injection, USP) 225 mg/30mL HDA Product Information Form
Click here to view Marcaine™ 0.75% (bupivacaine HCI Injection, USP) 225 mg/30mL Product ImagesShould you require additional information, please contact your Pfizer Hospital Trade National Account Director or Customer Service at 1-844-646-4398.
Sincerely,
Pfizer HospitalThe information provided is accurate at the time of this communication, and is subject to change without notice. Pfizer is not responsible for systems and databases external to Pfizer that may use this information in its current form or as superseded by future changes.
Please note that Average Wholesale Price (AWP) is set independently by compendia without input from Pfizer Inc. Pfizer plays no role in setting AWP and defers to compendia with respect to how AWP is determined.
Communication Type:
Product Changes
V#12114
-
05/14/2025
Title: ICU V#10917
Details:


Communication Type:
Supply Disruption
V#10917
Communication Type:
Temp Unavailable
V#10917
Communication Type:
Substitution Option
V#10917
-
05/14/2025
Title: ICU V#10642
Details:
Update on interruption of supply of pediatric endotracheal tubes April 2025
Attached is a customer listing for the past 6 month sales.
28 April 2025
Dear Valued Customers,
We appreciate your partnership and want to provide an important update regarding the ongoing supply of Portex™ pediatric endotracheal tubes due to the recent voluntary Field Action.
As previously communicated, the affected product codes are as below. Please note this issue only pertains to tube sizes: 2.0, 2.5mm, 3.0mm and 3.5mm; all other sizes in these ranges are not impacted. Smiths Medical have been manufacturing non-affected products since the end of October 2024 but are unfortunately still experiencing some backorder issues; this is due to the order quantities we are receiving relating to replacing all affected stock in the market whilst also supporting normal customer demand.
Smiths Medical have been manufacturing non-affected products since the end of October 2024 but are unfortunately still experiencing some backorder issues; this is due to the order quantities we are receiving relating to replacing all affected stock in the market whilst also supporting normal customer demand.
Please note that we are allocating stock fairly and expediting transit of manufactured products to our warehouses. Smiths Medical have added extra headcount and additional shifts in our factory to increase production of the impacted codes to try and reduce our recovery timeline.
We will provide regular updates on the supply situation via our regional commercial teams and welcome any questions you have regarding additional stock queries. For any questions related to the field action, Intubation ORAL/NASAL Endotracheal Tube FA2502-01, please continue to follow the directions shared in that communication.
Please do not duplicate orders as this will ultimately further delay the recovery timeline.
For further inquiries, please contact your customer service at (866) 829-9025 or customerservice@icumed.com or salesperson contact.Communication Type:
Backorder Notification/Update/Reports
V#10642
-
05/14/2025
Title: BD V#10125
Details:
Push Button Discontinuation Notice/ Customer Listing
Attached is a BD Pushbutton discontinuation letter.. Production has ceased for these 4 skus. Only sku 368656 has remaining inventory which we will continue to fill orders through the June timeframe. Suggested alternatives are listed. Please le me know if you have any questions.

Let me know if you have any questions!
Communication Type:
Discontinuation Notice
V#10125
Communication Type:
Substitution Option
V#10125
-
05/14/2025
Title: Pfizer V#12114
Details:
Pfizer Pharmaceuticals Memorial Day Hemophilia Holiday Schedule 2025
Dear Valued Customer:
We realize the importance of communicating our holiday schedule to customers. To support your planning efforts for Hemophilia orders, we are providing the following information for Pfizer operations during the upcoming holiday period. Please review this information closely.
Pfizer Holiday Schedule
Friday, May 23, 2025
Closing at 6:00pm EST
Monday, May 26, 2025
Closed
Pfizer Holiday Shipping Schedule
Orders Received
Expected Delivery By
Thursday, May 22, 2025 by 4:30pm EST
Friday May 23, 2025
Thursday, May 22, 2025 after 4:30pm EST
Wednesday, May 28, 2025
Friday, May 23, 2025
Wednesday, May 28, 2025
We will return to our standard business schedule on Tuesday, May 27, 2025. As always, Pfizer will make every effort to accommodate emergency needs as they arise.
If you have any questions about this schedule, please contact us at 1-888-440-8100.
Sincerely,
Kathy Dolan
Director, Customer ServiceCommunication Type:
Holiday Schedule
V#12114
-
05/14/2025
Title: Teleflex V#10538
Details:
Vascular Discontinuation Notice
Please see attached letter from Teleflex with items being discontinued. I have also attached a listing of customers who have purchased in the past 6 months.
Please reach out to Heather Burkett if you have any questions. Thanks,
Communication Type:
Discontinuation Notice
V#10538
-
05/14/2025
Title: BD V#10125
Details:
April Discontinuation Letter
Please see attached letter from BD on discontinuations. I have also attached the listing with customers who have purchased these in the past 6 months.
Please reach out to Heather Burkett if you have any questions. Thanks,
Across BD, we routinely evaluate our product portfolio to ensure we are continuing to meet the needs of our customers – and patients – around the globe. As a result, we will be making strategic decisions affecting our product portfolio. We believe these decisions can offer you a heightened customer experience by increasing the predictability of supply and demand, as well as the speed at which we can deliver products to you. In addition, we believe this will enable the introduction of new, innovative products.
To support your own planning, we want to give you notice of the following product changes. Below is a list of upcoming discontinuations - please refer to the appendix for the specific list of impacted products purchased by your organization, the discontinuation timeline, and recommended alternate BD products.* You and others in your organization may also receive additional information from BD about these upcoming discontinuations.
Material
Discontinued Date
Alaris Medley LVP Primary Admin Set
Select Alaris Medley LVP Primary Admin-15 mcrn Sets
Max Zero Extension Set
Various IV Access OEM - Private Label products
01/11/2025
01/11/2025
01/10/2025
01/10/2025
BD Pyxis™ CUBIE™ Mobile Dock
BD Pyxis™ CUBIE™ System Software
Effective immediately
Effective immediately
BD DifcoTM Inoculation Loops and Needles
9/30/2025
BD Clay Adams SurePrep Plain Capillary Tubes
5/30/2025
BD appreciates and values your business and looks forward to your continued interest in our products. While we recognize this change may impact your organization, we are confident that any potential short-term impact will be offset by our commitment to providing innovative products, solutions, and services.
To best serve you, all inquiries and requests should be directed through your local sales representatives or by contacting BD Customer Service at 1-844-823-5433.
Sincerely,
Joseph DiCandilo
Group Vice President
Channel Management, U.S. Region
Communication Type:
Discontinuation Notice
V#10125
-
05/13/2025
Title: Welch Allyn V#10834
Details:
BP Cuff Supply Update_Customer Communication_May9_2025
Concordance Team,
The remaining blood pressure cuffs from Welch Allyn V#10834 have removed from allocation in our system in accordance with the supplier’s attached announcement. A customer listing is attached for your reference.
Moving Forward:
- Welch Allyn has a second US based machine producing product coming online later this quarter which will be placed in their Skaneateles Falls, NY, facility.
- With these two new machines being in NY, they are confident that they are strategically positioned for growth, helping to ensure supply continuity and a pathway through the uncertainty surrounding tariffs.
Communication Type:
Allocation Notification/Update
V#10834
-
05/13/2025
Title: Megeden V#10423
Details: Vendor Item List Sales History_usage_made-in-China_MEDEGEN_8.12.24-5.12.25
Concordance Team,
Medegen sent the following message about their products that are sourced from China: “I want to provide you with an important update regarding select items Medegen sources from China. Due to recent disruptions in imports and pending tariff decisions, these items are now under limited supply. Once our current inventory is depleted, we will be unable to restock until the tariff situation is resolved. To ensure uninterrupted supply and minimize potential disruption, we strongly recommend reviewing and transitioning to the suggested alternative products as soon as possible. Although we are not currently out of stock, we are taking proactive steps to avoid any last-minute issues. We will continue to monitor the situation closely and provide updates as developments occur.”
The Chinese tariffs currently pose a continuous wait-and-see situation. Changes in tariffs and Medegen’s recent no-restocking decision could change any day.
- Medegen/Minigrip is not stating the items are discontinued at this point. We will have to address each item individually if a temp unavailable situation occurs.
- For the products in these files, what the supplier currently has in stock AND in transit from China, they anticipate it is equal to 3 months of inventory (in transit orders from China are not affected by the tariff).
- However, if customers start over-ordering, it could be depleted sooner.
- There are two usage reports attached for your reference…one for Medegen items and one for Minigrip.
Substitution Items:
- This communication has been shared with our product analyst who is working on loading any available subs to the Medegen/Minigrip items.
- This has been shared with the HCS team for them to seek sub opportunities.
- Medegen is actively working to find more alternative sources/products for these items.
- Medegen said the adhesive closure specimen bags are the most challenging to find an alternate source.
Communication Type:
Updated Supplier Information
V#10423
-
05/13/2025
Title: Minigrip V#13208
Details:
Vendor Item List Sales History_usage_made-in-China_MINIGRIP_8.12.24-5.12.25
Concordance Team,
Minigrip and Medegen sent the following message about their products that are sourced from China: “I want to provide you with an important update regarding select items Medegen sources from China. Due to recent disruptions in imports and pending tariff decisions, these items are now under limited supply. Once our current inventory is depleted, we will be unable to restock until the tariff situation is resolved. To ensure uninterrupted supply and minimize potential disruption, we strongly recommend reviewing and transitioning to the suggested alternative products as soon as possible. Although we are not currently out of stock, we are taking proactive steps to avoid any last-minute issues. We will continue to monitor the situation closely and provide updates as developments occur.”
The Chinese tariffs currently pose a continuous wait-and-see situation. Changes in tariffs and Medegen’s recent no-restocking decision could change any day.
- Medegen/Minigrip is not stating the items are discontinued at this point. We will have to address each item individually if a temp unavailable situation occurs.
- For the products in these files, what the supplier currently has in stock AND in transit from China, they anticipate it is equal to 3 months of inventory (in transit orders from China are not affected by the tariff).
- However, if customers start over-ordering, it could be depleted sooner.
- There are two usage reports attached for your reference…one for Medegen items and one for Minigrip.
Substitution Items:
- This communication has been shared with our product analyst who is working on loading any available subs to the Medegen/Minigrip items.
- This has been shared with the HCS team for them to seek sub opportunities.
- Medegen/Minigrip is actively working to find more alternative sources/products for these items.
- The supplier said the adhesive closure specimen bags are the most challenging to find an alternate source.
Communication Type:
Updated Supplier Information
V#13208
-
05/13/2025
Title: Masimo V#11799
Details:
Masimo Letter to Customers 5_9_2025
Concordance Team,
Masimo V#11799 recently experienced a cyberattack that has disrupted their daily activity. They are operational and processing/shipping orders, but because they have had to utilize some slower manual processes that have hindered their daily output, we could experience some short term delays.
The attached 5/9/25 Masimo letter was received yesterday, and below is some updated email communication that was delivered this week:
- We are now able to receive customer orders through EDI. Our customer service team is working through the backlog of orders. You will receive confirmation that EDI orders were received.
- If an order is placed in EDI and a confirmation is not received, please let me know.
- Any duplicate orders will automatically be cancelled.
- Orders we place on our factories for shipping are still being processed manually, so that automation has not yet been enabled. The team is working diligently to enable this as a next step.
The supplier also explained that the ten items that make up 80% of their revenue are in good supply. However, if we experience order fulfillment issues to our customers, please work with them to minimize the impact as much as possible through any suggested substitution items that we have available. For any extremely urgent orders, please email inquiries to TLUU@MASIMO.COM and JBLACK@MASIMO.COM.
Communication Type:
Supply Disruption
V#11799
- We are now able to receive customer orders through EDI. Our customer service team is working through the backlog of orders. You will receive confirmation that EDI orders were received.
-
05/12/2025
Title: Ansell V#11003
Details:
Tariff update
Dear Valued Partner,
Since our last communication, a 10% tariff has been levied on all countries with the exception of China. Further country-specific tariffs have been paused for 90 days—except for China, where a 125% tariff increase was implemented on top of the 20% tariff already imposed on March 4, 2025.
After reviewing the full impact across our portfolio, we are implementing a price increase of up to 10% for our Ansell medical glove styles, except for our vinyl gloves, which are sourced in China. All products sourced from China will have up to a 145% increase (inclusive of all previously communicated increases effective May 1). Previously communicated Canada and Mexico increases remain unchanged. All new increases will be effective June 23, 2025. We are striving to send updated price books to you by May 9, 2025.
Ansell’s long-standing and ongoing investments in a resilient and diversified supply chain - such as establishing alternate production sites, balancing regional sourcing, and strategically managing inventory - will help ensure consistent and reliable supply during this period of volatility. As a result, our gloves are manufactured in multiple countries to help minimize the tariff impacts and ensure supply chain resiliency. Additionally, we are in the process of establishing manufacturing locations in countries with lower impact for several of our Healthcare Safety Solutions. Nonetheless, we recognize that these increases have a meaningful impact on your cost of doing business, and we are here to support you and help customers navigate:
- Total Cost of Ownership. Our Ansell Services and tools are designed to help identify opportunities that may offset rising costs - through more efficient usage, injury risk reduction, and improved productivity – all while supporting the total cost of care delivery.
- Consistent Supply & Quality. Ansell has invested heavily in a resilient supply chain to ensure both supply and quality. Other suppliers may be challenged to offer the same level of assurance.
Finally, we understand that implementation of any change requires coordination, your time and resources. We appreciate the effort it takes your team to work through changes like this and are here to support you. We are committed to making this process as smooth as possible. The environment will continue to be dynamic, and we want you to know we will be regularly reassessing costs and pricing as tariffs and market conditions evolve. If you have any questions or would like to schedule a call to review in more detail, please feel free to reach out to the Ansell Representative supporting your account. We’ll continue to keep the lines of communication open throughout this process. Thank you again for your continued trust and partnership.
Kind Regards,
Sean Sweeney
Chief Commercial Officer, Americas
Ansell Healthcare LLC
111 Wood Avenue, Suite 210
Iselin, NJ 08830
United States
www.ansell.comCommunication Type:
Tarriff
V#11003
-
05/9/2025
Title: MidmarkV#10987
Details:
Midmark is offering your customers limited-time promotions across top-performing product lines—elevate their point of care ecosystem with serious savings. &nbs p;
SAVE NOW!
Exclusive savings on Midmark SolutionsNow's the time to act! Midmark is offering your customers limited-time promotions across top-performing product lines—elevate their point of care ecosystem with serious savings.
Explore these limited-time offers.
Point of Care Exam Room Promotion
Customers get $950 back or a Diagnostic Set ($2,300 Value) with a qualifying equipment purchase.
Examination Chair Trade-In Promotion
Rebates up to $400 + a Bonus $150 with delivery and trade-in removal services.
Customers save up to $500 when they trade in a sterilizer and purchase an eligible Midmark model.
Digital Vital Signs Device Promotion
Customers get $300 back or a FREE BP Cuff Kit ($249 Value) with eligible digital vital signs purchases.
Sterilizer Maintenance Kit Promotion
$225 Value—customers get a free maintenance kit when they register their new Midmark sterilizer.
For Dealers Only:
Exclusive Training Video PlaylistWe can help you start the conversation with customers. Watch brief overviews from our marketing team on each promotion.
We're contacting you because, as an existing or potential customer, we'd like to keep you informed about our products and solutions. To unsubscribe from similar communications, click here.
© 2025 Midmark Corporation, 60 Vista Dr, Versailles, Ohio 45380 USA
Communication Type:
End User Promo
V#10987
-
05/9/2025
Title: Abbott V#10575
Details:
attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File 5.8.25 Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Chad Truini; Sr. Business Analyst: email: chad.truini@abbott.com; phone: 614-624-0239
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Chad Truini
Sr. Business Analyst
Commercial Operations
Abbott Nutrition
3300 Stelzer Road
Columbus, OH 43219
O:
+1 614-624-0239
Communication Type:
Product Inventory Update
V#10575
-
05/8/2025
Title: Baxter V#10797
Details:
Baxter allocation update IV sets
Concordance Team,
Baxter removed the allocation from these IV sets and our system has been updated accordingly. A customer listed is attached for reference.

Communication Type:
Allocation Notification/Update
V#10797
-
05/8/2025
Title: Sage V#10229
Details:
Sage allocation update 9705 & 9717
Concordance Team,
Please be advised that these two Sage items have been removed from allocation:
Mfg#9705 C#110286
Mfg#9717 C#331682
Communication Type:
Allocation Notification/Update
V#10229
-
05/8/2025
Title: Kenvue V#10860
Details:
Vendor Item List Sales History 11.8.25-5.8.25
Concordance Team,
Kenvue V#10860 sent a notification that mfg#117749 (C#270642) Johnson’s Baby Lotion (25ml) is temporarily unavailable. This has been marked as such in our system effective today. Mfg #117468 (C#269997) Johnson’s Baby Lotion (50ml), is listed in VAI as a substitution.
Communication Type:
Temp Unavailable
V#10860
-
05/7/2025
Title: Attends V#10055
Details:
Attends & Comfees Websites & Social Media Sites 2025 .pdf (65)
Concordance Team,
Attends now has a new Attends & Comfees Digital Content Library that has many useful Digital PDF’s that your folks can access via ONE LINK. https://attindas.dcatalog.com/
There is also a PDF version attached.
Communication Type:
Product Catalog
V#10055
-
05/7/2025
Title: Nestle V#10837
Details:
Attached is the Nestle Inventory letter for this week.
- Benecalorie was removed
- Boost Chocolate was added
- Impact Peptide 1.5 remain on the list
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Experience our Support SiteCommunication Type:
Product Inventory Update
V#10183
-
05/6/2025
Title: Welch Allyn V#10834
Details:
WELCH ALLYN PROMOS THRU 6.30.25
Vendor Item List Sales History 9.6.24-5.5.25
Baxter WA RetinaVue Savings Offer Flyer_LR (1)
Baxter Vital Signs Promotional Flyer
Baxter Vital Signs Q2 2025 Promotional Part Numbers
Baxter Spot Vision Screener Promotional Flyer_LR
Baxter Resting ECG Promotional Flyer
Baxter Resting ECG item number list Q2
Baxter Q-Stress Promotional Flyer
Baxter Exam Tool Promotional Flyer_LR
Good morning, team.
Welch Allyn has the following promotional offers through the end of June 2025. A usage report is attached for reference.
Some of the attached flyers include the manufacturer item numbers but two do not. Refer to the Excel files for mfg item numbers for the ‘resting ecg’ and ‘vital signs’ promotions.
NEW CARDIO PROMOTIONS
- Resting ECGs
- $500 rebate on CP 150 or ELI 280
- $300 rebate on DCS
- $150 rebate on ELI 150c
- Q-Stress
- $2,000 rebate on Q-Stress System & TrackMaster
- $1,000 rebate on Q-Stress System on
NEW VITALS PROMOTION
- Spot Vital Signs 4400 Device, Connex Spot Monitor, Connex Vital Sign Monitor, & Connex Integrated Wall System
- $150 rebate
NEW RETINAVUE OFFER!
- RetinaVue Screener
- Up to 40% savings!
CONTINUATION OF EXAM TOOLS PROMOTION
- Exam Tools Trade up Offer- End-user rebate when "trading up" legacy Welch Allyn devices to our newer “BEST” configurations:
- Rebates on “BEST” configurations only
- Customers DO NOT need to send in old devices but need to provide a picture of what "they're trading in" on the rebate form
CONTINUATION OF VISION PROMOTIONS
- Spot Vision Screener
- $1,500 rebate with the purchase of the device & 1, 2, or 5-year SmartCare Service Plan
- This promo is for outpatient care offices only
- Spot Vision Screener Trade-Up
- $1,000 Spot Vision Screener Trade-Up until the end of June
Please see the attached promotional flyers. As always, contact your Welch Allyn product team for any help or assistance to bring these great offers to your customers!
End of Welch Allyn message.
Communication Type:
End User Promo
V#10834
-
05/6/2025
Title: Greiner Bio-One V#11403
Details:
Greiner Bio-One is pleased to announce the return of the Maveric Medical Blood Transfer Device to our blood collection accessories portfolio. This is item #M6018, which you might have previously marked as discontinued in your system.
If you have this item listed as discontinued, please take a moment to reactivate it, as it is now available once again through Greiner Bio-One. Here are the details for your reference:
M6018 - Maveric Medical Blood Transfer Device
- Single-packed
- Sterile
- Not made with natural rubber latex
-800 per case
Thank you for your attention to this update. We appreciate your continued partnership and look forward to providing you with the high-quality products you expect from Greiner Bio-One.
Communication Type:
New Product Launch
V#11403
-
05/6/2025
Title: Essity V#10272
Details:
Product Changes
Please review the attached product announcement for Essity Tena ProSkin Plus Underwear. This is a packing change only and does not impact uom, cost, or pallet configurations. Please share with appropriate members of your team.
Communication Type:
Product Changes
V#10272
-
05/6/2025
Title: Essity V#10272
Details:
Product Changes
Please review the attached Product Announcement regarding an item number change for the Cutimed Alginate family of products and forward to all operational areas for processing the change. We appreciate your support.
Communication Type:
Product Changes
V#10272
-
05/6/2025
Title: Baxter V#10797
Details:
Baxter allocation update notification
Concordance team,
Baxter provided an allocation update: “Please note the allocation on all 500mL and 1L pour bottles has been removed. This is occurring sooner than anticipated as the supply has improved sooner. The allocation will remain on the 250mL pour bottles for a few more weeks.”
The inventory analyst team has removed the following items from allocation and a customer listing is attached.
2F7113 CHS#662940
2F7114 CHS#605246
2F7123 CHS#662957
2F7124 CHS#605386
2F7154 CHS#645358
2F7184 CHS#505719
Communication Type:
Allocation Notification/Update
V#10797
-
05/5/2025
Title: Hartmann V#10739
Details:
Hartmann Sales Contacts - 2025
Concordance team,
Please see the attached Hartmann V#10739 sales contact list for your reference.
Communication Type:
Sales Roster
V#10739
-
Communication Type:
Product Inventory Update
V#10575
-
05/2/2025
Title: Midmark V#10987
Details:
See attached latest Tariff communication from Midmark concerning the impact of Tariffs. Please share internally as appropriate.
Let me know if you have any questions. Enjoy your weekend.
Best regards,
Communication Type:
Tarriff
V#10987
-
05/1/2025
Title: Busse V#10628
Details:
Busse Letter and 6 mo Usage
We want to inform you of an upcoming backorder on our Cat. No. 697 and 698 Procedure Drapes, which is expected to begin on May 5, 2025.
We understand how essential this product is to your operations and patient care, and we truly regret any disruption this may cause. Despite our best efforts to avoid delays, the back order is due to necessary upgrades to our drape packaging machine, including physical components, electrical systems, and software enhancements that are essential for proper production.
It’s important to note that these products are manufactured right here in the USA, not imported. This ensures we maintain strict quality standards and dependable supply, even as we work through these temporary challenges.
We are currently estimating a six-week lead time from the above date to replenish inventory, and we are doing everything we can to expedite this timeline.
Your trust and partnership are incredibly important to us. We remain committed to delivering the quality products and responsive service you expect from Busse Hospital Disposables. If there is anything we can do to assist your team during this time, please do not hesitate to reach out.
We sincerely apologize for the inconvenience and greatly appreciate your patience and continued support.
Sincerely,
Zach J Querci
Zach Querci
VP of Sales
Busse Hospital Disposables, Inc.
ZQuerci@Busseinc.com
Communication Type:
Supply Disruption
V#10628
-
Communication Type:
Sales Roster
V#10987
-
05/1/2025
Title: Cardinal V#10801
Details:
Enclosed is your Cardinal Health Products (“Products”) June 2025 distributor pricebook. The pricing is effective on June 1, 2025. We have included each Product’s packaging string for your reference. Once received, please update your system accordingly. You will receive future updates to your pricebook through our addendum process.
- Certain Product numbers end in a dash (-) or double dash (--). These dashes are a necessary component of our Product number. Not including the dash(es) could result in Product orders or rebates being delayed or rejected.
- Any Product with an * in the far right two columns of the attached pricebook indicate such Product is RX Only Product and Dropship Only Product. Cardinal Health Brand Products on the pricebook that are indicated as “Dropship Only” will be assessed freight which will be added to the applicable invoice. Please contact your sales representative with any questions.
- Open purchase orders for any Products subject to a price increase and on backorder as of June 1, 2025, will be cancelled. Your pricebook may not reflect a price increase for a SKU that is otherwise receiving an increase. Your backordered lines will still be canceled to retain parity for filling constrained SKUs. Backorder cancelations will take place in June.
- Price change timing is pursuant to Section 7.N (Extraordinary Events / Taxes) of the CPG Policies & Procedures.
- Pursuant to Section 7.M.iii of the CPG Policies & Procedures, Cardinal Health will issue an invoice for the increased value of your on-hand and in-transit inventory, reflected as of the effective date of the price change. Following the price change effective date, you will receive an inventory revaluation report from Cardinal Health, detailing the calculated quantity of on hand and in-transit inventory (in transit from Cardinal Health to your warehouse) impacted by the price change. Within 10 business days of receiving this report, you may provide Cardinal Health with a self-calculated report inventory on hand and in-transit. If a self-calculated report is not received, Cardinal Health shall proceed with the calculated inventory revaluation. All reports shall be submitted via email to your sales rep or account manager. The invoice for the increased value of inventory will not be eligible for a favorable prompt payment discount.
As always, we value your business and appreciate the opportunity to continue to provide you with quality products and services. If you have any questions, please contact us at CPGSupport@cardinalhealth.com or call customer service at (800) 635-6021.
Thank you,
Channel Partners Group
Communication Type:
Tarriff
V#10801
-
04/30/2025
Title: ICU Medical V#10917
Details:
New Product Launch
Please see below and attached for 6381. Please see attached for 6391.
Reach out to Heather Burkett if you have any questions.
ICU Medical is excited to announce the portfolio expansion with the launch of newly available enhanced
1000 mL Stericare SRB design to our portfolio for both 0.9% Sodium Chloride Irrigation USP and Sterile
Water for Irrigation USP. The enhanced bottle design, denoted with new product codes, will feature a
smaller 38 mm neck size and a tamper-evident break-band cap.
ICU Medical is in the process of adding these new product codes to our customer and GPO contracts. Please
begin your new product set-up process to best serve our mutual customers. ICU Medical anticipates product
availability and product shipment to begin 3Q2025.
Product Code
Product Description
GTIN
11-digit
Pseudo UPC
PUOM
CASE Size
Acquisition Case Price
6381
0.9% Sodium Chloride Irrigation USP, 1000 mL, Plastic Pour Bottle
Case -20850421008840
Unit - 00850421008938
85042100884
CASE
6
$42.36
6391
Sterile Water for Irrigation USP, 1000 mL Plastic Pour Bottle
Case – 20850421008796
Unit - 00850421008945
85042100879
CASE
6
$42.36
In order to answer any questions you may have, please contact myself or your assigned Distribution Manager for
more information or DistributorProductsPricing@icumed.com for any additional product attribute information
needed to successful create these new items within your system.
We sincerely appreciate your business and continued partnership with ICU Medical.
Communication Type:
New Product Launch
V#10917
-
04/30/2025
Title: Medline V#10605
Details:
Please see below and attached from Medline regarding the backorder on CX102/C#138813.
Item Backorder Notice
Hello,
This notice is to inform you that item CX102 is currently backordered.
We are working diligently with our vendors to get product in as soon as we can. We will follow up with ETA as we learn from
them. Currently, we anticipate being able to fill orders in mid June.
We are able to oer three substitute options in the meantime. The sub options are the CX302, MDS80529, and
MDS80529AS. See the chart below for more information regarding dierences between the three options. Please note
that while we currently have stock of the CX302 and MDS80529, this may change due to these items being oered as
substitute options.
1. The MDS80529 option has the same dimensions as the CX102
a. The biggest dierence is that the MDS80529 hamper comes unassembled
i. There are instructions in the case on how to build hamper
2. The MDS80529AS is the same hamper as option 1, however it is already preassembled
3. The CX302 hamper comes assembled, similar to the CX102
a. Biggest dierence is that CX302 is shorter – reference below chart for dimensions
Communication Type:
Backorder Notification/Update/Reports
V#10605
-
04/30/2025
Title: Pfizer V#12114
Details:
Introducing
Precedex Sodium Chloride Injection per Single-dose Container Products200 mcg/50 mL (NDC 0409-7838-24)
400 mcg/100 mL (NDC 0409-7853-24)
1,000 mcg/250 mL (NDC 0409-7875-12)April 30, 2025
Dear Valued Customer,
Great News! Pfizer Hospital is pleased to announce the availability of Precedex 200 mcg (50mL), 400 mcg (100mL) and 1,000 mcg (250mL) Sodium Chloride Injection per Single-dose Container.Inventory is available and ready for shipment to your distribution centers effective immediately.
Product Description
Unit of Sale NDC
Unit of Use NDC
List Price
Precedex 200 mcg/50 mL (4 mcg/mL) dexmedetomidine hydrochloride per
Single-Dose Container
(1 Case of 24 Containers)0409-7838-24
0409-7838-01
$672.00
Precedex 400 mcg/100 mL (4 mcg/mL) dexmedetomidine hydrochloride per
Single-Dose Container
(1 Case of 24 Containers)0409-7853-24
0409-7853-01
$1,200.00
Precedex 1,000 mcg/250 mL (4 mcg/mL) dexmedetomidine hydrochloride per
Single-Dose Container
(1 Case of 12 Containers)0409-7875-12
0409-7875-01
$2,545.20
Please click the links below to review important product information.
Click here to view USPI for Precedex
Click here to view SDS
Click here to view Precedex 200 mcg (50 mL) HDA Product Information Form
Click here to view Precedex 400 mcg (100 mL) HDA Product Information Form
Click here to view Precedex 1,000 mcg (250 mL) HDA Product Information Form
Click here to view Precedex 200 mcg (50 mL) Product Images
Click here to view Precedex 400 mcg (100 mL) Product Images
Click here to view Precedex 1,000 mcg (250 mL) Product ImagesShould you require additional information, please contact your Pfizer Hospital Trade National Account Director or Customer Service at 1-844-646-4398.
Communication Type:
New Product Launch
V#12114
-
04/30/2025
Title: ICU V#10917
Details:
Arterial Blood Sampling Supply

Communication Type:
Allocation Notification/Update
V#10917
Communication Type:
Backorder Notification/Update/Reports
V#10917
-
04/30/2025
Title: Perrigo V#13554
Details:
We are very excited to announce the launch of our new Rach Essentials toothbrush. Let me know if you are interested in the product. We are expecting our first shipment in late May, so single line POs are the best way to get some early. Let me know if you have any questions.
Regards,
Blake Campochiaro | Account Manager
Perrigo Direct, Inc.
Cell: 901-355-8264
Quality, Affordable Self-Care Products™
Communication Type:
New Product Launch
V#13554
-
04/29/2025
Title: Sage V#10229
Details:
Concordance Team,
The attached notice from Sage is about their discontinuation of mfg #7982 (C#168684). A 12-month usage report revealed that there have been no Concordance sales.
Communication Type:
Discontinuation Notice
V#10229
-
04/29/2025
Title: SC Johnson V#12219
Details:
GD8462 Concordance Healthcare Brochure041625
Concordance Team,
The attached brochure was also previously emailed by Don Allegretti of SC Johnson to a group of Concordance account managers with the following message: Attached is a PDF of all the current SKU's we have on the MMCAP's program with Concordance ( more coming in the near future ).
- Hand Soaps and Sanitizers, Floorcare products and General Surface cleaning products are the Core Segments
- Every G&E End User is buying products just like these every day
- Our # 1 Place to win everyday is K-12 and Higher Ed with our Floorcare and Hand Soaps.
- For Large opportunities, we have 35 Direct Sales people to help you in the field.
- I will be your main point of contact. Donald T. Allegretti: DTAllegr@scj.com. Please feel free to pass this document on to all your customers and contact me if you have any questions.
Communication Type:
Product Flyer
V#12219
-
04/29/2025
Title: Kenvue V#10860
Details:
Concordance Team,
Kenvue Brands V#10860 provided the attachment to display NEW items. If a customer has demand, send a request to the Item Maintenance team to set up the item(s) in our system.
Communication Type:
Product Flyer
V#10860
-
04/29/2025
Title: Welch Allyn V#10834
Details:
BP Cuff Supply Update_Customer Communication_Mar28_2025
Concordance Team,
As a follow-up to the 4/3/25 posting, the fully-configured cuffs for both soft and vinyl have been removed from allocation/Temp Unav. and the original customer listing is attached.
Communication Type:
Allocation Notification/Update
V#10834
-
04/28/2025
Title: Teleflex V#10538
Details:
Teleflex Central Access 2025 SKU Discontinuation
Customer usage and letter with C#'s are attached here.
Dear Valued Customer,
Teleflex is updating our Arrow™ Central Access product lines in the United States to support healthcare systems
in complying with clinical practice guidelines, standardizing vascular access products, and ensuring a stable and
consistent product supply. To better serve your needs and align with clinical best practices, we are streamlining
our Arrow™ Central Venous Catheter (CVC), Multi-Lumen Access Catheter (MAC), Percutaneous Sheath
Introducer (PSI), Large-Bore Central Venous Catheter (LB) and Acute Hemodialysis (AHD) kits.
SKUs listed in Table 1 will be discontinued over the next 6-12 months. While the typical discontinuation period is
6 months, Teleflex has extended this timeframe to up to 12 months to better support your transition planning.
Clinical alternatives have been identified for all affected SKUs and are provided below.
After the 6-12 month discontinuation period or when stock is depleted, orders will be redirected to the best
clinical alternative to meet your vascular access needs. A specific discontinuation date will be communicated by
late 2025 after evaluating availability of materials and components to ensure consistent supply.Communication Type:
Discontinuation Notice
V#10538
-
04/28/2025
Title: BSN V#10231
Details:
TEMPORARYTEMPORARY Product Change from Gypsona® and OCL® to Specialist®
As part of our commitment at Essity to delivering quality products and solutions, we continually review and innovate to enhance your experience. We are also dedicated to promoting health and hygiene while responsibly managing our environmental resources.
Health and hygiene are essential to people's well-being, and our products and services enable people all over the
world to live healthy and dignified lives.We are reaching out to inform you of some updates to our Gypsona® and OCL® portfolio.
What is Changing: We are updating the manufacturing processes for Gypsona® and OCL® which will enable us to continue delivering the high-quality products you expect while enhancing our sustainable practices.
Historically, Gypsona® and OCL® have been manufactured using solvent-based processes. As of 2025, we will transition to a water-based manufacturing process.
How It Benefits You: Extensive acceptance testing with clinicians across North and South America has been conducted to ensure the new manufacturing process's stability and reliability. This helps ensure that while the composition of the product may have changed, the performance of those products is still befitting of the Gypsona® and OCL® brands.
What Is The Timeline For This Transition: The transition is scheduled to occur in August 2025. Until then, we will offer our Specialist® assortment. This product line has always used our water-based manufacturing process.
Specialist® Plaster of Paris utilizes a time-tested formula that produces a consistent plaster that is uniformly coated
and adhered to the gauze. This unique formula offers minimal plaster loss to ensure maximum strength and a
smooth cast finish. It is available in rolls and slabs with extra fast setting time (2-4 minutes) and fast setting time
(5-8 minutes).
Due to the temporary transition to Specialist®, there will be a change in the order codes. Please refer to the attached list of affected products.
We greatly appreciated your continued support and trust in our brands and products. If you have any questions or
need further clarification, please don't hesitate to reach out to our customer service team.Communication Type:
Backorder Notification/Update/Reports
V#10231
-
04/28/2025
Title: GP V#10978
Details:
Concordance Team,
Please see the attached Georgia Pacific sales roster for future use.
Communication Type:
Sales Roster
V#10978
-
04/27/2025
Title: Roche Diagnostics V#10603
Details:
2025 Roche Promotions Grid_Concordance
Roche Diagnostics NPC Inside Sales 2025 Roster
Vendor Item List Sales History 4.25.24-4.24.25
Concordance Team,
Roche Diagnostics V#10603 is offering the four promos which are valid through 12/31/25.
Coag:
1) Roche material # 06298176001. (c#178346) CoaguChek XS 3-Box Starter Kit
Includes: CoaguChek XS Meter + 3 boxes CoaguChek 48-ct test strips
2) Roche material # 08468699001. (c#263528) CoaguChek XS 6-Box Starter Kit.
Includes CoaguChek XS Meter + 6 boxes CoaguChek 24-ct test strips
UA:
3) Roche material number 10467665001. (c#410769) Urisys 1100 Analyzer Barcode Scanner Starter Kit.
Includes: Urisys 1100 analyzer + Urisys 1100 Barcode Scanner
4) Roche material number 03260763602. (c#122433) Urisys 1100 Analyzer Chemstrip 10MD Starter Kit
Includes: Urisys 1100 analyzer + (6-boxes) Chemstrip 10MD strips + (1 box) Chemstrip UA Calibration strips
These are drop-ship, not inventoried kits.
- Attached is a PDF file with the promo description.
- Attached is a PDF roster/map for the Roche Diagnostics Inside Sales Team.
- They are handling all Coag now as well as all Chemstrip/Micral urinalysis, Accutrend, and glucose accounts outside of the largest 300 in the country.
- The national account manager at Roche requests that you start with this team, and if there is a glucose customer that they don't cover, they can direct you to the appropriate field rep.
Communication Type:
End User Promo
V#10603
-
04/27/2025
Title: Procter & Gamble V#10097
Details:
Concordance Team,
Procter & Gamble V#10097 does not publish a sales roster, but below are two P&G professionals who can assist in getting you connected with the right sales contact:

PLEASE NOTE: Brian Biebuyck will retire at the end of May. Meg Cross is his boss. She will let me know when they hire Brian's replacement, and then I will create an updated post.
Communication Type:
Sales Roster
V#10097
-
04/25/2025
Title: NestleV#10183
Details:
Nestlé Health Science is committed to providing high-quality, science-based nutrition formulas. In the spirit of continuous improvement, we would like to share the following updates to our product portfolio.
Extensive HA® - Ingredient & Pallet Configuration Update
Extensive HA® infant formula is undergoing a minor ingredient statement update. Due to a change in the supplier of DHA oil, the source of DHA in Extensive HA® will now be reflected as SCHIZOCHYTRIUM oil (formerly C. COHNII oil) in the ingredient statement. The overall nutrient profile of the formula will remain the same with the new oil source.
In addition to the ingredient statement change, Extensive HA® cases will now be packed on pallets to minimize shipping damage. This change reduces the number of cases per pallet from 128 to 112 cases.
It is important to note that only the DHA oil source & pallet case count are changing. Product codes will remain the same. The new formula will flow into the market as inventories of the old formula are depleted. The estimated in-market dates are dependent on our current inventory supply and are subject to change.
Product Description
No ChangesProduct Code
Case UPC/GTIN Code
Est.
In-Market Date
No Code Changes
Extensive HA® Unflavored 6 x 400 g
5000048519
00050000485192
May 2025
Should you have any questions or require additional information, please work with your Nestlé Distributor Manager, or call our Consumer Service Center at 1-800-422-2752 that is staffed with Registered Dietitian Nutritionists (RDN). Additional product information and support tools can be found on our Medical Hub at www.nestlemedicalhub.com. Thank you for your continued business, and we appreciate your understanding and look forward to serving you with our improved products!
Sincerely,
Felicia Belle
Vice President, Head of Sales
Nestlé Health Science, US
Communication Type:
Product Changes
V#10183
-
Communication Type:
Product Inventory Update
V#10575
-
04/25/2025
Title: Cardinal V#10801
Details:
Transitioning/Discontinuing Kits 6 month usage
Transitioning/Discontinuing Trays
Kit #
Description
Decision
Presource Kit Cross
Lumbar Puncture
1017
1017 LUMBAR PUNCTURE 18G
available until runout
26-LP6SFA
1032-
1032 LUMBAR PUNCTURE 20G
26-LP1SFDA
1048-
1048 LUMBAR PUNCTURE PED
26-LP2SFDA
7039-
7039 LUMBAR PUNCTURE INFANT
26-LP2SFDA
7500
7500 LUM PUNC INFANT W/MONO
discontinued
26-LP2SFDA
Amnio
97051
TRAY AMNIOCENTESIS PROCEDURAL
discontinued
29-AM1SFA
Bone Marrow Biopsy Trays
8881847118
BN MAR BIOP/ASP NDL 11X4
discontinued
31-BM1SF / 31-CR1SF
8881847084
BN MAR BIOP/ASP NDL 8 X 4
discontinued
31-BM1SF
8881847019
BN MAR BIOP/ASP TR 11X416G
discontinued
31-BM2SF / 31-CR2SF
8881847035
BN MAR BIOP/ASP NDL 8X416G
discontinued
31-BM2SF
8881847167
BN MAR BIOP TR W/16GA NDL
discontinued
31-BM3SF (not direct cross)
8881847993
BONE MARROW BSIC BIOPSY TRY
discontinued
31-BM4COA
Bone Marrow Needles only
8881247111
BN MAR BIOP/ASP NDL 11X4LL
available until runout
BMNJ11X4
8881247087
BN MAR BIOP/ASP NDL 8X4 LL
SPP99BM8A
8881247129
BN MAR BIOP/ASP NDL 13X2.5LL
SPP99BM13A
8881245164
16G SINGLE I NEEDLE
BMNI15GX2
8881247137
13G X 3IN BN MARR NEEDLE
SPP99BM13A
Trocar Trays
8888565507
THORACOSTOMY PROC TRAY
discontinued
12-5507
8888565028
TROCAR CATH KIT 12FR
discontinued
12-5507
8888565036
TROCAR CATH KIT 20FR
discontinued
12-5507
8888565044
TROCAR CATH KIT 28FR
discontinued
12-5507
Centesis Trays
5016
PARACENTESIS TRAY
discontinued
30CE2SFAFA
5014
5014 THORACENTESIS TRAY
discontinued
30CE2SFAFA
8888567032
PNEUMOTHORAX TRAY
discontinue
No Cross
Turkel Cath
M2355
THORACENTESIS NEEDLE
discontinued
No cross
M2359
TUBING SET WITH ROLLER CLAMP
discontinued
502036002
Dennis Mahoney
Key Account Manager, Channel Partners Group
7000 Cardinal Place, Dublin, OH 43017
(781) 974 – 7218 mobile
Communication Type:
Discontinuation Notice
V#10801
-
04/25/2025
Title: Baxter V#10797
Details:
Baxter allocation update 4.22.25
Concordance Team,
Baxter has removed the following items from allocation. They have been removed from allocation in our system as well, and a customer listing is attached.

Communication Type:
Allocation Notification/Update
V#10797
-
04/24/2025
Title: Integra Lifesciences V#10439
Details:
I wanted to share some very important communication regarding the status of any open MediHoney orders you may have currently pending. Unfortunately, the distribution of MediHoney is voluntarily on hold. We are evaluating the impact of non-conformances identified at our distribution centers for MediHoney as part of the standard quality process and should have more information on the timelines in about one month. We will provide an update at that time or hopefully sooner. Please note that this is not a recall and all MediHoney product in circulation is completely safe.
Mary, our teams are fully committed and working feverishly internally to ensure product is released as soon as possible because we do understand the impact the hold can make on our customers but most importantly the patients we serve who rely on MediHoney the most.
Attached is a letter for your reference. There is a portion at the bottom of the letter with communication we hope is helpful for you to share with your direct customers as you receive inquiries on status. Upon review please let us know if you have any further questions or concerns. We value Concordance’s invaluable partnership, Mary, and appreciate your patience. I will keep you updated as soon as we receive an update on the release. Thank you for your support!
Warm regards,
Steph
Stephanie Williams
Associate Director, Distributor Relations
Tissue Technologies
609.936.4616 office • 609-213-9131 cell • 609.750.4277 fax
stephanie.williams@integralife.com
Integra • 1100 Campus Road, Princeton, NJ 08540
www.integralife.comCommunication Type:
Backorder Notification/Update/Reports
V#10493
-
04/23/2025
Title: ICU Medical V#10917
Details:
ICU Medical IV Solution Product Availability and Allocation Update
Dear Valued Customer,
Since October 1, 2024, ICU Medical has been solely focused on ensuring our committed and loyal customers have the IV Solutions
they have needed to serve their patients amid the latest IV Solutions market event. To meet the ongoing IV Solution needs of our
customers, ICU Medical implemented mandatory overtime, moved to increase staffing, and added manufacturing shifts throughout Q4
’24 and Q1 ’25, which have resulted in a significant increase in IV Solution production above pre-hurricane levels.
To further strengthen our future market presence and supply resiliency, we are in-process of finalizing our joint venture with Otsuka
Pharmaceutical Factory, Inc., which will deliver immediate and ongoing investment, supply resiliency, choice and meaningful
innovation to this critically essential product category.
As a result of the actions cited above, we are pleased to announce that effective May 1, 2025, we will be removing all
protective allocations across our US IV Solutions portfolio for historical committed customers with the following exceptions
noted in the table below.
Should you have additional questions or need assistance, please contact our Customer Care Department at 1-866-829-9025 or email
ProductAvailability@icumed.com. Thank you again for your business and continued support of ICU Medical products.Communication Type:
Allocation Notification/Update
V#10917
-
04/23/2025
Title: Bayer V#11854
Details:
Discontinuation Notice EOL on 3 Sku's
Please see attached notices from Bayer Healthcare, LLC.
MEDRAD® Mark V ProVis Syringe Kits (150-FT-Q, 200 FT-Q)- Channel Partners (1)
CTP-200-FLS EOL Notification Partner Letter (1)
Last 6 Months Customer Listing with Sales and Units
Please reach out to Heather Burkett if you have any questions. Thanks,
Communication Type:
Discontinuation Notice
V#11854
-
04/22/2025
Title: Midmark V#10978
Details:
Team Concordance,
We really appreciate you making the effort to make the trip to Versailles! As Jason stated, we had a chance to collaborate, learn how we can help each other grow along with getting to know each other better. I have attached a youtube video you can share with your customers who are considering a Midmark experience trip. It provides a little overview of what they would be coming to see. I have also included the US Access board information that we discussed. Hope everyone has a great weekend, we look forward to working together soon.
Designing better care.™
Gerard Palopoli
Region Director
Northeast Region
Medical Products & ServicesCell: (516) 220-7755
Communication Type:
Product Inventory Update
V#10978
-
04/22/2025
Title: Graham Medical V#11028(10030)
Details:
Behavioral Health Category Sheet (002)
Concordance Team,
Please refer to the below Graham Medical Catalog link along with the four (4) attachments (V#10030-NDC supplier - Graham Medical). You will find the following:
1. Medical catalog link: 2024-Graham-Medical-Catalog.pdf
2. Graham Medical sales territory map (attached) ---see your sales contact name, phone number, & email address
3. ASC flyer (attached)
4. IDN brochure (attached)
3. Behavior Health Category Sheet: patient identification (attached)
****Graham Medical will provide samples upon request****
Communication Type:
Sales Roster
V#11028(10030)
Communication Type:
Product Flyer
V#11028(10030)
Communication Type:
Product Catalog
V#11028(10030)
-
04/20/2025
Title: GE Medical V#10126
Details:
GE V#10126 email about discontinued Items 4.18.25
Vendor Item List Sales History 10.18.24-4.18.25
Concordance Team,
GE Medical V#10126 discontinued a long list of items. Of those items, the attached Excel file 'Vendor Item List Sales History' (usage) is filtered to show the short list of those discontinued items for which we had usage over the past six months.
Communication Type:
Discontinuation Notice
V#10126
-
04/17/2025
Title: Medtronic V#10255
Details:
Medtronic_ACM Sales Roster 8-19-24
Medtronic_Medical Surgical Remote Sales Roster_All OUs_Q3 FY24
Medtronic_U.S. ASI Sales Roster 3.28.23 - Combined Sharable
Concordance Team,
Please find attached the current Medtronic sales rosters (3 files)
Communication Type:
Sales Roster
V#10255
-
04/17/2025
Title: ICU V#10917
Details:
Joint Venture - ICU Medical and Otsuka Pharmaceutical Factory Inc
Dear Valued Customer,
In mid-November, we publicly announced our Joint Venture with Otsuka Pharmaceutical Factory, Inc. (OPF), a global leader in IV solutions manufacturing. As previously communicated, we are committed to maintaining transparency and ensuring a smooth transition as we move toward the operational launch of the joint venture, which is anticipated to be completed by May 1, 2025. Enclosed you will find a formal communication outlining important information regarding the upcoming changes.
Should you have any questions, our Customer Service Team is available to assist you:
• Email: CustomerService@icumed.com
• Phone: 866-829-9025
• Hours of Operation: 7:00 AM – 6:00 PM CST
We thank you for your continued partnership and look forward to supporting you throughout this transition.
Best regards,
Customer Excellence
Communication Type:
Updated Supplier Information
V#10917
-
04/17/2025
Title: Audit Microcontrols V#12466
Details:
NEW PRODUCT LAUNCH- Linearity FD Bilirubin
We have just launched a new product, K742M-5 Linearity FD Bilirubin. Please see the attached sell sheet and updated price list for your reference.
Please make a note:
The K701M-SUPP Linearity FD Bilirubin Supplement was a FOC product that was automatically shipped with each K701M-5 General Chemistry kit. It was an analyte that didn’t play well with the others and so the manufacturer pulled it out into a supplement kit. Recently, they decided to make Bilirubin its own product, for purchase. So, nothing to discontinue in your system. However, if there are questions as to why a customer didn’t receive the FOC kit, this is the reasoning. They will no longer get a FOC bilirubin product.
Please reach out to Heather Burkett if you have any questions.
Please see attached Sales sheets and price list.
Communication Type:
New Product Launch
V#12466
-
04/17/2025
Title: Molnlycke V#10008
Details:
2025 Hibiclens 32 oz - Gallon July 1 2025 Discontinuation - Distribution Notification
Vendor Item List Sales History 10.17.24-4.17.25
Concordance Team,
MESSAGE FROM MOLNLYCKE V#10008: After careful internal evaluation and strategic review of our Hibiclens product portfolio, Molnlycke has made the decision to discontinue two SKUs within this product line. The SKUs being discontinued are:
- 57532 HibiClens 32oz bottles
- 57591 HibiClens 1-gallon bottles
Please accept this notification and the attached formal letter from our Hibiclens Global Marketing Director as the 90-day notification of this discontinuation. These products will be discontinued effective July 1, 2025. Fulfillment will be based on previous 12-month usage or until inventory is depleted. This product discontinuation reflects our strategic alignment to exit bulk volume products. We remain deeply committed to our mutual customers with comprehensive support to the remainder of our portfolio.
Communication Type:
Discontinuation Notice
V#10008
-
04/17/2025
Title: Pfizer V#12114
Details:
NEW PRODUCT LAUNCH- PAXLOVID™ (nirmatrelvir tablets, ritonavir tablets)
Pfizer Announces the Introduction of PAXLOVID™ (nirmatrelvir tablets, ritonavir tablets), co-packaged for oral use, for patients with severe renal impairment
Dear Wholesaler:
Pfizer, Inc is pleased to announce the introduction of PAXLOVID™ (nirmatrelvir tablets, ritonavir tablets), for commercial use for patients with severe renal impairment. Please click the links below to review important product information.
Click here to view the Customer Letter
Click here to view Prescribing Information
Click here to view Safety Data Sheet - Nirmatrelvir
Click here to view Safety Data Sheet - Ritonavir
Click here to view HDA Product Information Form
Click here to view Label Images
Click here to view Product Fact Sheet
Should you require additional information regarding this product, please contact your National Account Director or call Customer Service at 1-800-533-4535.
Sincerely,
Parmjit Agarwal
Vice President
Channel ManagementCommunication Type:
New Product Launch
V#12114
-
04/17/2025
Title: Audit Microcontrols V#12466
Details:
NEW PRODUCT LAUNCH-
We have recently launched another new product, K739M-10 Linearity FD Traumatic Brain Injury (TBI). Please find attached a sell sheet and a price list reflecting this new addition.
Please reach out to Heather Burkett with any questions. Thanks,
Communication Type:
New Product Launch
V#12466
-
04/17/2025
Title: Pfizer V#12114
Details:
Price Increase (Tariff)- effective May 15th
April 15, 2025
Dear Valued Customer,
Effective 12:01 AM EDT, May 15, 2025, prices for various Pfizer Hospital products will be revised. Revisions include one hundred and sixteen (116) price revisions (excluding private label products) on the Excel file.
As of the release of this notice and until May 15, 2025 all submitted orders are subject to review and may be cancelled if quantities ordered are deemed unusual or excessive by Pfizer.
Please click the link below to review important price information.
Click here to view the Excel file
Pfizer is not responsible for systems and databases external to Pfizer that may use this information in its current form or as superseded by future changes.
Price information is confidential and proprietary of Pfizer and is disclosed to recipients solely for their own use. Recipients are reminded that disclosure of this document is prohibited and may subject customer to a claim(s) of breach of contract and/or unfair business practices.
Should you have any questions or need additional information please contact your Trade National Account Director.
Communication Type:
Tarriff
V#12114
-
04/17/2025
Title: Sekisui V#10161
Details:
NEW PRODUCT LAUNCH
We are launching a new product that we would like to load with Concordance.
Item 1091: OSOM RSV Test; 25 tests +2 for QC inside per EA; 18 EA per CS;$189/EA; $3,402/CS.
Product documentation and images are attached. Please let me know if anything further is needed to support loading this item.

Communication Type:
New Product Launch
V#10161
-
04/16/2025
Title: Welch Allyn V#10834
Details:
Concordance Team,
Attached is a Welch Allyn sales rep roster for your reference. Message from the supplier: "Our teams are split by market segment and geographies. The reps with the title of territory managers are our Primary Care team and the ones with the title of account executives are responsible for the Acute Care market. If you or your teams have a question of coverage for a specific territory or a specific let me know and I can get that sorted out for you.”Communication Type:
Sales Roster
V#10834
-
04/16/2025
Title: Health-o-Meter V#10075
Details:
Hi Concordance Team,
We have product updates below for the 844KL and the 2595KL, as well as a new product to be added to your system. I have attached your item spreadsheet for the new product, and ‘ve also attached the updated Spec Sheets. Please let me know if I can provide any additional information.
844KL:
- Updated Power Source: 2 3-Volt Lithium batteries 2 AAA batteries (included)
2595KL:
- Added Functions:
Variable auto-off Time
Enabling unit view with unit of measure locked
Hold/Release
Reweigh
Pre-Tare
Recall
Everlock®
- Update EMR Connectivity from USB to say via optional Pelstar wireless technology
Below is the product announcement for the new 2595KG:
Health o meter Professional Scales Introduces Enhanced Features for Digital Chair Scale
Health o meter® Professional Scales is excited to introduce upgraded features for the 2595KL Digital Chair Scale. The 2595KL is an accurate weighing solution for patients with limited mobility, featuring flip arm and foot rests for easier patient access and wheels that provide easy mobility to and from the patient’s location. New upgrades include Variable Auto Off Time, a new Unit View setting, Hold/Release functionality, Everlock® (permanent unit lock), and availability as a new kilograms-only version, 2595KG. To purchase upgraded 2595KL or new 2595KG, visit authorized Health o meter® Professional Scales distributors, and for additional product information, visit homscales.com/2595KL or call 1-800-815-6615.
thanks,
Kelly Virzi
Sales and Marketing Specialist
Pelstar LLC
Health o meter® Professional Scales
Bridge Healthcare
9500 West 55th Street
McCook, IL 60525-7110 USA
Direct Phone: 708-377-0549
weigheasier®
Communication Type:
New Product Launch
V#10075
-
04/16/2025
Title: Qosina V#10623
Details:
Dear Amy,
I'm here with good news: at this time, we have no plans to increase unit prices despite shifting tariffs. Our scale and global footprint will enable us to help mitigate the current international trade disruptions. As we navigate the evolving tariff landscape together, we will continue to keep you apprised of any potential pricing or invoicing changes.
I want to personally reassure you that Qosina remains your steadfast partner during these times of tariff uncertainty. We understand price stability is crucial for your planning, and we're committed to providing that security. Our diverse and strategic sourcing approach positions us well to minimize tariff impacts. We've built strong supplier relationships across multiple regions, allowing us to adapt sourcing strategies as needed while maintaining product quality and availability.
Our robust inventory is ready to support your needs. We encourage you to please schedule a conversation with your sales representative via their calendar link below. They're prepared to help you secure the components you need during this time.
Sales regions:
Northeast US – Justin Mantrana
Northwest and Southwest US – Keith Lewis
Southeast US – Allison Achille
Latin America and Asia – Kelly Peritore
All other international regions – Alyssa Kolb
Thank you for your trust in Qosina. We value our partnership and are here for you—now and in the future.
Warm regards,
Lee Pochter
Lee Pochter
CEO
Qosina Corp.
2002-Q Orville Drive North
Ronkonkoma, NY 11779 USA
T: +1 (631) 242-3000
Email: lpochter@qosina.com
www.qosina.comCommunication Type:
Tarriff
V#10623
-
04/16/2025
Title: Ansell V#11003
Details:
April 16, 2025
Dear Valued Partner,
Since our last communication, a 10% across-the-board tariff has taken effect on April 5. Further country-specific tariffs have been paused for 90 days—except for China, where a 125% tariff increase was implemented on top of the 20% tariff already imposed on March 4.
After reviewing the full impact across our portfolio, we are implementing a price increase of up to 10% across all styles, except for our small segment of products impacted by the China tariffs, where a 90% increase will apply (in addition to the previously communicated increase effective May 1). Previously communicated Canada and Mexico increases remain unchanged.
For KleenGuard™ and Kimtech™ products, a 104% increase will be applied to China manufactured products in the absence of a May 1 increase.
All new increases will be effective on orders dated to ship starting June 1, 2025. Updated price books will begin to be shared in late April and received by all customers by the third week in May.
We understand the evolving tariff impacts across products and industries and Ansell is here to support you.- China Sourced Alternatives. Over 90% of our products are sourced outside of China. If you are currently purchasing products made in China from Ansell or from another supplier, Ansell has alternatives. Reach out to your Ansell Channel or Territory Manager who can advise you on Ansell options.
- ANSELLGuardian®. Our safety assessment tool is designed to help identify opportunities that may offset rising costs - through more efficient usage, injury risk reduction, and improved productivity. In addition, Ansell’s comprehensive portfolio includes value-driven styles for everyday protection alongside our most advanced, industry-leading solutions all of which offer longer lasting protection. Contact your Ansell Channel or Territory manager who can help you meet your safety, cost, and performance goals without compromise.
- Consistent Supply & Quality. Ansell has invested heavily in a resilient supply chain to ensure both supply and quality. Many other suppliers may be challenged to offer the same level of assurance. If you have concerns about your existing supplier, reach out to your Ansell Channel or Territory Manager.
We understand that pricing implementation requires coordination and time on your end. The environment continues to be increasingly dynamic and therefore, we will continue to keep the lines of communication open throughout this process. We appreciate the effort it takes to work through changes like this. Our team is committed and here to support you.
Thank you again for your continued trust and partnership.
Kind Regards,
Sean Sweeney
Chief Commercial OfficerAmericas
Communication Type:
Tarriff
V#11003
-
04/15/2025
Title: Kenvue V#10860
Details:
Kenvue_Copy of Concordance Band-Aid Codes
Vendor Item List Sales History 4.14.24-4.14.25
Concordance Team,
Band-Aid has discontinued three (3) codes. Please see the attached file from Kenvue V#10860 which includes suggested replacement items with pricing in case it is determined that they should be set up in our system.
There was no usage over the past 6 months. The attached usage report is for the trailing 12 months.
Communication Type:
Discontinuation Notice
V#10860
-
04/15/2025
Title: Midmark V#10987
Details:
See attached letter regarding Midmark’s statement on Tariffs. Please share within your organization as appropriate.
Thank you…!
Communication Type:
Tarriff
V#10987
-
04/15/2025
Title: Medegen V#10423
Details:
Vendor Item List Sales History 10.15.24-4.15.25
Concordance Team,
Medegen item #112MBX (C#377551) has become obsolete. A 6-month usage report is attached but reveals that only 4 cases were sold.
Medegen's suggested replacement:
112M- Bag: Lau 30.5X41 1.1Ml Yel Pr 250/Cs Price - $43.37 per case.
The only difference between these 2 items is item number 112MBX is folded and prepack 50 each vs item number 112M is 10 rolls of 25.
Notify the Item Maintenance team to set up the this item in the system if there is customer demand.
Communication Type:
Discontinuation Notice
V#10423
-
04/11/2025
Title: Georgia Pacific V#10978
Details:
Concordance Team,
Georgia Pacific V#10978 sent a notification that the following items are on allocation. See the monthly allocation by DC. They have been added onto allocation in our system and a customer listing is attached.

Communication Type:
Allocation Notification/Update
V#10978
-
04/10/2025
Title: Nurse Assist V#10080
Details:
product discontinuation letter for 2 SKUs with the suggested alternative items. 6 Month Customer usage Can you please cancel these POs in your system, and I will do the same on our side. Please advise if you will be transitioning demand to the new SKUs and will be placing new Pos. Please confirm that you have closed those items on your orders and discontinued the items in your system.
Reach out with any questions, thanks.
Best Regards,
Michele Smith
Customer Service
SteriCare Solutions
4409 Haltom Road
Haltom City, TX 76117
800.649.6800 x120
This information may be confidential and/or privileged. Use of this information by anyone other than the intended recipient is prohibited. If you receive this in error, please inform the sender and remove any record of this message.
**PLEASE NOTE WE HAVE A NEW E-MAIL FOR ORDERS ONLY**
PLEASE SEND ORDERS TO ORDERS@NURSEASSIST.COM
Communication Type:
Discontinuation Notice
V#10080
-
04/10/2025
Title: BD V#10125
Details:
Please see attached allocation tool from BD if needed.
Please note: This includes contact info on allocated items and who can approve changes, escalations and increases from each business unit. It also includes a sales roster for each business unit.
Please let Heather Burkett know if you have any questions.
Communication Type:
Allocation Notification/Update
V#10125
-
04/10/2025
Title: BSN V#10231
Details:
Discontinuation Notice- Wax Coated Paper Bucket
Please see notification on C#202149 if needed. Thanks,

Communication Type:
Discontinuation Notice
V#10231
-
04/10/2025
Title: Pfizer V#12114
Details:
Please see letter below on Pfizer Award if needed. Thanks,
Pfizer Inc.
275 North Field Drive
Lake Forest, IL 60045
April 9, 2025
Pfizer Awarded Healthcare Industry Resilience Collaborative (HIRC)
Diamond Resiliency Badge
Dear Valued Customer,
We are proud to share an exciting achievement—Pfizer has become the first pharmaceutical manufacturer to receive the prestigious Diamond Resiliency Badge from the Healthcare Industry Resilience Collaborative (HIRC) for our sterile injectables portfolio. This recognition is a testament to our continued investments in supply chain excellence and underscores our commitment to reliable supply and availability of generic sterile injectables for the long-term.
The HIRC Resiliency Badge Program serves as a symbol of a supplier’s ability to withstand disruptions and deliver critical supplies, ensuring continuity of patient care, illustrating Pfizer’s high-quality manufacturing practices and global supply chain network. For hospitals that utilize the HIRC program for strategic supplier management, this badge demonstrates:
- Proof of Resiliency & Operational Health – Shows Pfizer’s ability to navigate supply chain challenges and deliver critical products without disruptions.
- Manufacturing Strengths & Innovations – Reflects Pfizer’s commitment to manufacturing best practices, innovation, and continuous improvement to respond efficiently to dynamic market needs.
- Continuity of Patient Care – Reinforces Pfizer’s role as a strategic partner in supporting the healthcare industry’s mission to provide uninterrupted patient care.
HIRC, a non-profit organization dedicated to strengthening the healthcare supply chain, awards the Diamond Resiliency Badge through a rigorous, evidence-based assessment across multiple criteria. This evaluation measures supplier resilience across critical areas including Demand Planning, Inventory Management, Logistics, Supply Chain Visibility, Supplier Management, Risk Management & Contingency Planning, and Operational Health. This independent review, conducted by PricewaterhouseCoopers, was determined by product category assessment.
With the largest and broadest portfolio of sterile injectable products in the U.S., Pfizer Hospital takes pride in being recognized for our operational excellence and dedication to providing the highest quality products to our customers and, ultimately, to our patients. We encourage you to join us by registering for an upcoming Customer Webinar on Tuesday, April 15 or Wednesday, April 16 where we will share more information and insights on the HIRC Diamond Resiliency Badge and how this can help reinforce your purchasing decisions with high-quality strategic suppliers.
Thank you for being a valued part of our journey. We look forward to continuing to serve you with the highest standards of quality and reliability.
Sincerely,
Najah Sampson
U.S. Hospital Lead
Communication Type:
Updated Supplier Information
V#12114
-
04/10/2025
Title: Dr Easy V#10400
Details:
New Product Launch below

Communication Type:
New Product Launch
V#10400
-
04/10/2025
Title: Molnlycke V#10008
Details:
Molnlycke - Alldress Dressing Availability Distribution Partner Update April 10 2025
Vendor Item List Sales History 10.10.25-4.10.25
Concordance Team,
Please see below from Molnlycke V#10008 about three item codes that are being discontinued BUT they anticipate having product available through the end of 2025, if not longer. A 6-month usage file and the supplier's formal announcement is attached.

Communication Type:
Discontinuation Notice
V#10008
-
04/10/2025
Title: Baxter V#10797
Details:
Baxter allocation email 4.9.25
Concordance team,
Baxter update: The vast majority of small volume IV products have been removed from allocation. The product that remains on allocation is the 2B0043. (C#772533)
Please see the attached Baxter letter for the item listing. Updates have been made in our system by the inventory analyst team accordingly.
Communication Type:
Allocation Notification/Update
V#10797
-
04/9/2025
Title: Sekisui V#10161
Details:
New Product Launch- 3 Tests
Please see attachments below for pictures:
FDA EUA Authorization Letter for Combo
We currently expect to have product available to officially launch this product no later than May 1, 2025 but we will be in communication with you if this date changes either earlier or later.
Item MTRX-C19+FLU-25PK: Metrix COVID/Flu Test Kit 25 pk; 25 tests per KT; Price per kit $972 (Sekisui sells KT at minimum)
Item MTRX-RDR-GEN2: Metrix Reader Gen 2; 1 Reader EA; Price per EA $28 (Sekisui sells EA at minimum)
Item MTRX-C19+FLU-B1: Metrix kit (2) and Reader (1) bundle; 1 bundle per EA; Price per kit $1960 (Sekisui sells EA at minimum)
Current dating on the test kit is 12 months so we are requesting that the items be loaded as drop ship only initially.
Communication Type:
New Product Launch
V#10161
-
04/9/2025
Title: ICU V#10642
Details:
Reference: Level 1™ Trauma and Normoflo™ Fluid Warming Sets Supply Disruption
Dear Valued ICU Medical Customer,
This letter is to inform you that we are continuing to experience a supply shortage of the following products which affects the production of the following Level 1 trauma and Normoflo fluid warming sets.
We are continuing to produce these disposables and will fill customer orders. However, the inventory remains in allocation, which will result in delays in filling your complete order. We continue to work closely with the supplier to improve production in the coming months. Further updates will be provided as appropriate.
We sincerely appreciate your use of these products, and we apologize for any inconvenience experienced by patients, customers, or our distributor partners. Please contact your sales representative or customer services at 1-800-258-5361 with questions.
Sincerely,
Dhairav Dalal
Marketing Director – Temperature ManagementCommunication Type:
Allocation Notification/Update
V#10642
-
04/9/2025
Title: BD V#10125
Details:
Update on end-user BD IV Fluids allocations going forward-
Dear Valued Customers,
The purpose of this letter is to provide an update on end-user BD IV Fluids allocations going forward.
BD is committed to supporting U.S. health care providers in delivering patient care while strengthening supply chain resilience through domestic manufacturing of critical medical products. As a part of this commitment, BD requires visibility into its future demand to adequately service its customers.
Starting May 1, 2025, BD will focus on servicing only our committed customer base regardless of level of commitment for our IV Fluids offering. This approach ensures that we can accurately plan future availability and maintain continuity of care for our customers as usage volumes are detailed through committed agreements.
Commitments are executed through an IV Fluids Purchasing Agreement or an IV Fluids Letter of Commitment. These agreements can be arranged through your BD sales representative. If you would like to request a representative or have other questions, please contact BD Customer Care at 1-844-823-5433 or visit www.bd.com/en-us/support.
Thank you for your continued supportCommunication Type:
Product Changes
V#10125
Communication Type:
Product Inventory Update
V#10125
Communication Type:
Product SKU List
V#10125
Communication Type:
Allocation Notification/Update
V#10125
-
04/8/2025
Title: Busse V#10628
Details:
UPCOMING BO
Please see attached letter explaining the reason for the upcoming backorder. 6 month usage
In roughly 2 weeks’ time, we will be on BO on our item 696 Sterile Polylined Drape/300each to a case which is expected to begin April 14, 2025.
We are currently estimating a six-week lead time from this date to replenish inventory.
We sincerely apologize for the inconvenience and greatly appreciate your patience and continued support.
Sincerely,
Lucy Fiore
Busse Hospital Disposables
75 Arkay Drive
Hauppauge, NY 11788
800-645-6526 EXT. 244
Communication Type:
Supply Disruption
V#10628
-
04/8/2025
Title: Baxter V#10797
Details:
Customer Letter_Discontinuation Notice of Select IV and Irrigation Solutions
Vendor Item List Sales History 10.4.24-4.4.25
Concordance Team,
Per the attached Customer Letter that addresses 11 Baxter item codes being discontinued between April and October 2025, the following 9 Baxter items have been discontinued and placed into item inactivation in our system by the IM team. This inactivation in our system includes some items on their list that have Estimated Stockout Dates of May, June and October 2025, because there have not been recent sales. This leaves S#102788 (Mfg #2B1614X) and S#605188 (Mfg #2B2073Q) active for now. Baxter still has inventory available for both items.
S#700302 Mfg #2B1373Q
S#156962 Mfg #2B2103Q
S#156969 Mfg #2B7477 (IM previously addressed)
S#954651 Mfg #2B7116
S#100475 Mfg #2B1134X
S#852277 Mfg #2B1644X
S#824086 Mfg #2B1664X
S#961979 Mfg #2B2224X
S#300155 Mfg #2D5613Q
Communication Type:
Discontinuation Notice
V#10797
-
04/8/2025
Title: Nestle V#10183
Details:
wanted to share the attached communication and Letter with you. After reviewing it, if you have any questions or need further clarification, please do not hesitate to reach out to your Nestlé Distributor Manager, who will be more than happy to assist you.
Have a fantastic day ahead!
Amy Sauber, RDN, LDN
Manager, Sales Support Team
Nestlé Health Science U.S.
CONFIDENTIALITY NOTIFICATION: This email message and any attached documents are intended only for the use of the intended recipient(s) and may contain information that is confidential. If you have received this email in error, please notify the above sender and delete it from your computer.
Communication Type:
Product Inventory Update
V#10183
Communication Type:
Product Update
V#10183
-
04/8/2025
Title: Cardinal V#10801
Details:
See attached letter around a discontinuation in our incontinence portfolio. 6 month usage file
Our recommended substitute is the exact same item and quantity in a case, just a different packing count in the case.
Thank you!
Dennis
Dennis Mahoney
Key Account Manager, Channel Partners Group
7000 Cardinal Place, Dublin, OH 43017
(781) 974 – 7218 mobile
Communication Type:
Discontinuation Notice
V#10801
-
04/7/2025
Title: Amsino V#10385
Details:
Subject: Important Notice: Tariff Impact on Amsino Pricing
Dear Valued Customer,
We would like to share an important update regarding recent changes in trade policy that will affect pricing for Amsino products.
As you may be aware, the Trump Administration announced on April 2, the implementation of reciprocal tariffs on all imported products from China and more than 120 countries. This is an increase to the previously implemented tariffs that have already been enacted on medical products.
There are no exceptions for medical device products under these new tariffs, including all Amsino products.
Effective June 1, 2025, prices for all Amsino products manufactured outside of North America will be updated to reflect the impacts of these new tariffs. Please expect further communication from your Amsino Account Manager detailing the specific pricing adjustments for your products soon.
We understand that this adjustment may present challenges for your business, and we are committed to addressing them as best as possible.
This situation continues to evolve, and Amsino remains committed to expanding our onshoring and nearshoring manufacturing capabilities such as our recent acquisition of MedXL in Canada. Through these investments, we are reinforcing the values our customers rely on most: uncompromised quality and long-term supply assurance. We are actively working to identify long-term solutions to address these changes and will keep you informed as new developments arise.
We deeply value your partnership and look forward to continuing to serve you as a trusted supplier. If you have any questions or concerns, please do not hesitate to reach out to your Account Manager.
Yours sincerely,Communication Type:
Tarriff
V#10385
-
04/7/2025
Title: Midmark V#10987
Details:
631 Exam Table
Midmark®
631 Procedure ChairSay hello to the first and only US Access Board compliant procedure chair.
Healthcare is changing—more patients, more in-office procedures and a growing demand for accessibility. The Midmark 631 lowers to 17" while maintaining a 39" high seat height. Using US Access Board (USAB) compliant Patient Support Rails, patients can transfer safely onto the 28-inch wide compliant transfer surface. Additionally, customers can properly support the patient's thigh, knee and calf during lower body exams and procedures using USAB compliant knee crutches. The 631 is also compatible with patient lifts, helping ensure safe transfer with little interference. The future of patient care starts now.
Support the future of patient-centered, accessible in-office procedures—starting today.Interested in making a purchase?
Tell us how to contact you and we’ll be in touch with more details.
We're contacting you because, as an existing or potential customer, we'd like to keep you informed about our products and solutions. To unsubscribe from similar communications, click here.
© 2025 Midmark Corporation, 60 Vista Dr, Versailles, Ohio 45380 USA
Communication Type:
New Product Launch
V#10987
-
04/7/2025
Title: Ansell V#11003
Details:
Tariff
Ansell Healthcare Products, LLC
----
111 Wood Avenue South
Iselin, NJ 08830
United States
T. + 1 732 345 5400
T. + 1 732 219 5114
https://url.avanan.click/v2/r01/___www.ansell.com___.YXAzOmNvbmNvcmRhbmNlaHM6YTpvOjg0YmQxMjdlNjQ2MTdjMWNhNGRjM2FhOWEzZDA3OGYzOjc6MzZlMzphOTY5Zjc4NGQ1MzU4ZWE3NWM4OWFmOWIzNzBjODVmYTQ1MDMyMzY0ZWRjOWVkOWE3MmRjNTFkOWE5Y2IwNjdlOnQ6VDpGApril 4, 2025
Dear Valued Partner,
We want to update you on the latest trade developments and what they mean for our industry. On April 2, President Trump announced new tariffs that will take effect in the coming days. Beginning April 5, 2025, an across-the-board tariff will be imposed on all U.S. imports. Then, on April 9, additional tariffs will be applied to many countries, replacing the initial across-the-board tariff but adding to any existing tariffs. Some of these tariffs are significant, and we recognize the broader implications for businesses across the globe.
We understand the seriousness of this situation and want to assure you that we are working tirelessly to assess the impact and develop a measured response. With trade policies shifting rapidly, we recognize that this environment remains unpredictable and creates volatility in many industries. While these tariffs will create challenges, Ansell has long invested in a resilient and diversified supply chain to help minimize disruptions. This foundation positions us to navigate the changes while continuing to provide reliable supply and support to our customers.
That said, we know that these tariffs will have considerable cost implications and in turn, pricing impacts. While we cannot eliminate the impact, our team is working diligently to determine the best path forward and explore all possible options to manage these changes thoughtfully.
We will provide updates as soon as we have more details, including exact pricing adjustments and implementation timing, where applicable. Transparency remains our priority, and we are committed to keeping you informed every step of the way. Should you have any questions in the meantime, please don’t hesitate to reach out to your Ansell Channel Manager or Territory Manager. We appreciate your trust and partnership as we work through these evolving circumstances together.Kind Regards,
Sean Sweeney
Chief Commercial OfficerAmericas
Ansell, ® and ™ are trademarks owned by Ansell Limited or one of its affiliates, except as noted.
© 2024 Ansell Limited. All Rights Reserved.Communication Type:
Tarriff
V#11003
-
04/7/2025
Title: Sage V#10229
Details:
Sage allocation notice mfg 9705 & 9717
CONCORDANCE TEAM,
By the direction of Sage Products, the following two CHG items have been put on allocation in our system so that only current Sage usage customers can receive product:
Mfg#9705 C#110286
Mfg#9717 C#331682
REASON:
- Medline’s inventory of product codes MSC096CHG and MSC099CHG is constrained
- Recently a Concordance rep informed Sage that a Concordance customer who currently buys Medline CHG from Concordance is switching to Sage products due to unavailable Medline products
- Sage wants to prevent the customer or other Medline CHG users from overtaking the Sage products and wants to ensure that current Sage customers remain whole and are not impacted by Medline’s product code constraint
IMPORTANT:
- If other customers who were previously buying Medline CHG from Concordance would like to start ordering Sage or use Sage as a sub, please only allow a drop ship PO to come through
- Send drop ship requests to SMC@stryker.com while cc’ing Dina Wiersma at dina.wiersma@stryker.com
- For any account that wants to convert to Sage, please email Dina directly for her review and approval
Communication Type:
Allocation Notification/Update
V#10229
-
04/4/2025
Title: Nestle V#10183
Details:
Attached is the weekly inventory letter for Nestle. 6 month usage
MCT Oil was added
Compleat Pediatric Peptide 1.0 250 is still on the list
Thickenup Clear Packets is still on the list
Benecalorie was removed
Communication Type:
Discontinuation Notice
V#10183
Communication Type:
Product Inventory Update
V#10183
-
04/4/2025
Title: Baxter V#10797
Details:
Customer Letter_Discontinuation Notice of Select IV and Irrigation Solutions
Vendor Item List Sales History 10.4.24-4.4.25
CONCORDANCE TEAM,
Baxter V#10797 announced in the attached letter that there is a list of products being discontinued. The estimated stockout dates are included. Please also see the 6-month usage report for your reference.
Communication Type:
Discontinuation Notice
V#10797
-
04/4/2025
Title: Procter and Gamble V#10097
Details:
Vendor Item List Sales History 10.3.24-4.3.25
Concordance Team,
Procter and Gamble has discontinued mfg #17116 (C#131731). Please refer to the different subs listed in VAI, including an HSC product. A 6-month usage file of this discontinued product is attached.
Communication Type:
Discontinuation Notice
V#10097
-
04/4/2025
Title: Baxter V#10797
Details:
Customer Listing- Allocation Percentage Changes
Concordance Team,
Baxter V#10797 has announced allocation changes, and Casey notified that updates have been made in our system.
- The PDF letter includes allocation changes on 4/01/25 and expected changes into the future
- Attached Excel sheets are only of the items with allocation changes on 4/01/25 per the supplier’s attached PDF notification
- Excel sheet: Customer Listing- items that were removed from allocation
- Excel sheet: Customer Listing- Allocation Percentage Changes – customers who are using the items which percentages increased.
Communication Type:
Allocation Notification/Update
V#10797
-
04/4/2025
Title: J&J V#10196
Details:
US HN Customer Letter_FINAL_042025
Copy of JJMT - Product Discontinuation Notice - April 2025
Vendor Item List Sales History 10.4.24-4.4.25
Concordance Team,
J&J sent the attached pdf discontinuation notice that explains that certain items are being discontinued in multiple phases. Our Concordance item numbers have been added in column J on the Excel file that they provided which includes a "target last sale date" column. Also attached is our 6-month usage report.
Communication Type:
Discontinuation Notice
V#10196
-
04/4/2025
Title: Vantive V#13578
Details:
Acute Care Allocations Update- 4-1-25- Final
Concordance Team,
The following Vantive V#13578 items have been removed from allocation and a customer listing of those using them is attached.
CHS#161755 mfg#107501S
CHS#337981 mfg#110244S
CHS#255275 mfg#114905
Communication Type:
Discontinuation Notice
V#13578
-
04/4/2025
Title: Convatec V#10695
Details:
Convatec AWC Post Acute 04.03.25
Convatec Critical Care Roster 04.01.25
Concordance Team,
Please find attached the current Convatec sales rosters.
Communication Type:
Sales Roster
V#10695
-
04/3/2025
Title: Medline V#10605
Details:
Midstream Collection Kits- Temp Unavailable
Please see notification below from Medline regarding Midstream Collection Kits and sub.
C#100142 is temp unavailable in VAI. Please note there are backorders in KNOX for Wayne County and Cookeville Regional. Please note backorders in RIPL for Boone Memorial and Pikeville. This will not be ordered from Medline. Attached is a customer listing with usage and spend for the past 6 months.
The suggested sub is C#875138 from Medline. Please reach out if you have any questions. Thanks,
________________________________________________________________________________________________________
Hello Distributor Partners,
We are still experiencing an extended backorder on our Deluxe Midstream Collection Kit (item DYND30212).
We won't receive any additional inventory until mid to late August, and this item is now on full allocation.
In the meantime, please consider the following alternate options:
DYND30224 - KIT, MIDSTREAM, HANDLE/BZK, 4OZ CUP, VALUE
- This item features a collar instead of a full funnel and a standard cup that is not 95kPa certified.
- It comes in 100 EA/CS, whereas the original item is 40 EA/CS.
- See below for an overview of the suggested substitute.

SPXB9521O - CONTAINER, 90ML, FUNNEL, WIPE, STERIL
- This distributed substitute features a 95kPa cup.
Please note lead times for the above item are dependent on quantity, so please get an order in ASAP if your customer would like this item.
Communication Type:
Backorder Notification/Update/Reports
V#10605
Communication Type:
Substitution Option
V#10605
-
04/3/2025
Title: Steris V#10863
Details:
As a Distributor of impacted SKUs, action is required.
- Update item descriptions and GTINs
- Update website to include new item descriptions, images, and instructions for use (IFU).
- Until further notice, Distributors may only sell items 2D73QH, 2D74QH, and 2D79QH as a complete case.
- Why? Starting March 31, 2025, cleared product will begin shipping.
- VERIFY RESI-TEST SLIDE-THRU Cleaning Process Protein Indicator (LCC101) will include cleared labeling.
- Brush item SKUs 2D73QH, 2D74QH, and 2D79QH labeling will also introduce the cleared product name, VERIFY RESI-TEST SLIDE-THRU Brush on the case label.
- On the outside of each brush case, there will be a letter indicating that brushes can be used as is with the VERIFY RESI-TEST SLIDE-THRU CPP Indicator under the enclosed IFU. Individual brush and box labeling will be updated upon inventory depletion.
Impacted SKUs
SKU
UOM
New Item Description
New GTIN
Other important change
New Image
LCC101
BX
VERIFY RESI-TEST SLIDE-THRU Cleaning Process Protein Indicator
10724995231900
Updated product name, labeling, and expanded viewing box for negative control tests
*Note – item not available to all distributors; distribution status not changing
Steris Verify ResiTest Slide Thru1323 w TMs cmyk.tif | Powered by Box
2D73QH
CA
VERIFY RESI-TEST SLIDE-THRU Brush (1.0-1.2mm)
50724995231915
Product may be sold by CASE only
https://steriscorp.box.com/s/8bhwsu2ebyw5iuxsfu7nwbx6gibka4i4
2D74QH
CA
VERIFY RESI-TEST SLIDE-THRU Brush (1.5-2.6mm)
50724995231922
Product may be sold by CASE only
https://steriscorp.box.com/s/nwftp17z529oix41wax2kgeokied85yt
2D79QH
CA
VERIFY RESI-TEST SLIDE-THRU Brush (2.8-5.0mm)
50724995231939
Product may be sold by CASE only
https://steriscorp.box.com/s/8bhwsu2ebyw5iuxsfu7nwbx6gibka4i4
There are no changes to unit of measures, quantity, or acquisition costs currently.
We appreciate your immediate attention to ensure changes are in place on March 31, 2025.
If you have questions regarding these changes, please contact me at cindy_tanski@steris.com or at 440-445-8508.
Thank you,
Cindy Tanski
Director, Distributor Channel | Healthcare
STERIS
5960 Heisley Road, Mentor, OH 44060
Direct phone: 440-445-8508
Email: cindy_tanski@steris.com
Communication Type:
Product Changes
V#10863
Communication Type:
Label Changes
V#10863
-
04/3/2025
Title: Welch Allyn V#10834
Details:
BP Cuff Supply Update_Customer Communication_Mar28_2025
Welch Allyn Cuff SKUs by Cuff Type_33125
CONCORDANCE TEAM,
Welch Allyn announced that their highest volume BP cuffs, FlexiPort Standardization/RAW Soft are now removed from allocation. As shown in the below Matrix, they anticipate removing the FlexiPort Fully-Configured Soft and Full-Configured Vinyl from allocation on April 14, 2025. We will let you know when they formally announce this.
- See the attached PDF letter which includes this table.
- See the attached customer listing for reference.
- Welch Allyn installed a U.S. production line in Q1 2025 which has been fully operational since March 14 and will install a second new production line by the end of May 2025 which will further enhance their ability to achieve and maintain normal lead times.

Communication Type:
Allocation Notification/Update
V#10834
-
04/2/2025
Title: Health-O-Meter V#10075
Details:
BridgeAir_Single_Patient_Use_F&B_20250311
BridgeStar_System_F&B_20250319
CONCORDANCE TEAM,
Health-O-Meter sent the following message about new products from the Bridge Healthcare line. Please also refer to the documents that are linked to this post. If you receive customer demand for any new products, reach out to the Concordance Item Maintenance team to set up the items in the system.
Bridge Healthcare by Pelstar Launches New BridgeAIR+STAR air-assisted transfer mattresses
Pelstar LLC announces several new products expanding Bridge Healthcare’s BridgeSTAR portfolio. New variations of BridgeAIR+STAR air-assisted transfer mattresses are now available in multiple sizes featuring an easy to clean, breathable fabric that supports a favorable environment for skin health. As with other BridgeAIR+STAR mattresses, these products provide significant reduction in the forces required to laterally transfer, turn, and reposition patients. Another addition to the BridgeSTAR portfolio is a new offloading wedge set that features a wedge with an anchor tail. For a complete safe turning and repositioning system, facilities should also utilize a BreatheDry absorbent pad, now including a new 37”x56” size option. For more information on the BridgeSTAR system and the full range of Bridge Healthcare’s Safe Patient Handling solutions, visit www.bridgehcusa.com
SKU
Description
PS37SPUO
BridgeAIR™+STAR-O, Mattress Single Patient Use Full, 37”x56” with Velcro straps
PS40SPULT
BridgeAIR™+STAR-LT, Mattress Single Patient Use Full, 40”x80” with Velcro straps
PS40SPULTS
BridgeAIR™+STAR-LT Mattress Single Patient Use Half, 40”x49” with Velcro straps
PS50SPULT
BridgeAIR™+STAR-LT, Mattress Single Patient Use Full, 50”x 80” with Velcro straps
BH-BD3756
BreatheDRY™ Standard 37” x 56”, BridgeAIR™ Absorbent Quick Dry Breathable Pad
PS-W15119T
BridgeSTAR™ Turning/ Repositioning Wedge with Tail, 30°, 15" x 11" x 9.5"
Communication Type:
New Product Launch
V#10075
-
03/28/2025
Title: MicroCare Medical V#10201
Details:
Product Rebrand Letter Catalog
MicroCare is currently undergoing a product rebrand. We are changing our product name from the “Pro” line to “Spec Clean” Additionally, we are adding “JIL’s” or job information language on the label. The new imagery will bring a fresh new look to our products.
Please note, our formula has not changed.
We will be rolling out the new products throughout Q1 and Q2 of the 2025 fiscal year. We will include bottle tags and box stuffers in each case that will explain this change to customers.
For your convenience, I’ve attached our announce letter as well as our new product catalog.
Please feel free to contact me with any questions.
Thanks,
Charlie Campbell
Key Account Manager
6120 East 58th Avenue
,
Commerce City
,
CO
,
80022
Communication Type:
Product Catalog
V#10201
Communication Type:
Product Changes
V#10201
-
03/28/2025
Title: Abbott V#10575
Details:
the attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Joe
Communication Type:
Product Inventory Update
V#10575
-
Communication Type:
Product Inventory Update
Nestle V#10183
-
03/27/2025
Title: CryoConceptsV#12777
Details:
CryoConcepts is excited to offer a Q2 Spiff. Attached is program details. To help you in your sales efforts, we have been able to stock items in most of our DC’s to make it easier to sell by the each/kit.
- $25 for every Histofreezer FLEX, CryOmega, or CryoClear kit sold during the 2nd quarter!
- SPIFF runs from Tuesday, April 1st – Monday, June 30th
- Payout per Concordance Spiff payment terms
Please reach out to with any questions to myself or the contacts listed in the attachment. OR stop by their booth at CONTRIBUTE.
Thank you for all you efforts in the field and HAPPY SELLINGCommunication Type:
Rep Spiff
CryoConcepts V#12777
-
03/27/2025
Title: Abbott V#10575
Details:
We are sharing with all of our distributor partners the attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Joe
Joe Corey
Sr. Business Analyst
Abbott Nutrition
Commercial Operations
Abbott
3300 Stelzer Rd.
RP 2-3
Columbus, OH 43219
O:
+1 614-624-6600
F:
+1 614-727-3896
Communication Type:
Product Inventory Update
Abbott V#10575
-
03/27/2025
Title: GE Medical V#10126
Details:
DINACLICK-compatibility-guide_-1 Black and -5 Gray Connectors_JB02857XX
Vendor Item List Sales History 9.26.25-3.26.25_GE Medical
2024-11-25 ISO 80369-5 Standard Frequently Asked Questions JB11177US
Copy of GEHC BP Cuffs DINACLICK Dash-1 to Dash-5 part numbers
Concordance Team,
This is from GE: This is regarding GE HealthCare CRITIKON™ non-invasive blood pressure cuffs.
We are transitioning from the ISO 80369-1 DINACLICKTM connection system to the ISO 80369-5 DINACLICKTM connection system.
The timeline for this transition has begun and we looking to be fully transitioned by early to mid-2025.
The “Dash 1” cuffs (per the attached excel file) will no longer be available after April 15th, 2025.
If you have questions or there is some other action that needs to take place, please let me know.
Please review the attached FAQs, the compatibility guide, and the part number list.
Communication Type:
Discontinuation Notice
V#10126
-
03/26/2025
Title: Dukal V#10239
Details:
Tariff notice
Dear Valued Channel Partner,
This email is to inform you that due to significant increases in government-imposed tariffs, Dukal is forced to revise the attached prices products, effective May 1, 2025.
We realize that these changes may pose challenges for you and your business. Please feel free to reach out to us if you have any questions or need further assistance.
Please confirm receipt and let us know if you have any questions or need further information.
Thank you for your continued partnership and understanding.
Communication Type:
Tarriff
Dukal V#10239
-
03/26/2025
Title: Medela V#10262
Details:
Dear Trading Partners,
Please see the attached Discontinuation Notice for the following material:
Medela Item#
Description
68064
Milk Storage Bags 180ml 25ct EN
87124
Tender Care Hydrogel Gel Pads 100ct EN
87123NA
Tender Care Hydrogel Gel Pads 4ct EN
Thank you.
dd
Communication Type:
Discontinuation Notice
Medela V#10262
-
Communication Type:
Product Catalog
Sloan V#10319
-
03/25/2025
Title: Nordson V#10428
Details:
Tariff Notice
March 4, 2025
RE: Tariffs on Imports from Mexico
Dear Valued Customer,
Effective March 4, 2025, the US Government has imposed an additional 25% tariff on all products imported from Mexico. This change will impact products we manufacture in Mexico on your behalf.
Since these changes are substantial and could be resolved in a timely manner, we will pause all shipments from our manufacturing facilities in Mexico for a period of two weeks. Should these changes remain after such time, we will commence shipping products from our facility to a bonded warehouse from which you will be able to manage importation and transportation. More details regarding logistical considerations will be forthcoming.
We greatly appreciate your business and patience during these volatile times.
Sincerely,
Daniel P. Lazas
Vice President, Sales & Marketing
NordsonMEDICAL.comCommunication Type:
Tarriff
Nordson V#10428
-
03/25/2025
Title: Cardinal V#10801
Details:
Tariff Notice
Please see below for the latest communication from Cardinal Health Leadership regarding tariffs.
Effective March 4, 2025, at 12:01 a.m. ET, the White House enacted:
- A 25% tariff on goods from Canada
- A 25% tariff on goods from Mexico
- An amended tariff on goods from China, from 10 to 20%
- These import tariffs will impact how we manufacture and source the products you need and will lead to price increases to ensure product availability.
- This will not be unique to us — our industry will face this challenge.
- Where possible, we have already moved quickly to increase U.S. manufacturing capacity and exit markets with greater tariff risks, but we operate in a highly regulated environment that requires time to shift supply chains.
- We will do what we can to minimize impacts of the new trade tariffs, but we are a low margin business and corresponding price increases should be expected.
- We will provide more information about product category pricing impacts in the coming weeks.
- In the meantime, we will continue to be deeply engaged with trade associations to advocate on behalf of our industry and customers, sharing our concerns with the Administration, and fighting on your behalf to minimize disruptions to our healthcare supply chain.
- For current information on what is known about the new tariffs to-date, visit: 2025 Tariff Updates
As I receive additional updates or information on this evolving situation, I will connect with you; however, please do not hesitate to reach out with any questions in the meantime.
Kindest Regards,
Dennis
Dennis Mahoney
Key Account Manager, Channel Partners Group
7000 Cardinal Place, Dublin, OH 43017
(781) 974 – 7218 mobile
Communication Type:
Tarriff
Cardinal V#10801
-
03/25/2025
Title: Ansell V#11003
Details:
Tarriff Notice
January 31, 2025
Dear Valued Partner,
As part of our commitment to transparency, we’re writing to update you on the evolving situation regarding potential tariffs. As you may be aware, the new US Administration is currently considering imposing additional tariffs on US imports. Our team is closely monitoring this situation, and as the specific policy changes are officially implemented, we are planning a coordinated effort across functions to address the impact of these tariffs through pricing adjustments and sourcing strategies.
We understand that these developments may cause concerns, and we want to assure you that Ansell is actively working to manage these challenges. Should these tariffs come to fruition, we will need to implement price adjustments to offset the increased costs. While we do not yet know the exact impact, we will act quickly to quantify the effect and communicate and minimize the price impact.
As we navigate this uncertainty, it’s important to note that Ansell’s supply chain remains resilient. Over the years, we’ve made significant investments to diversify and strengthen our manufacturing footprint, which has enabled us to minimize reliance on any single region. As a result, and due to our scale, we are uniquely positioned to help mitigate regional impacts and, above all ensure supply chain reliability.
For example, when the Office of US Trade Representatives modified China tariffs in September, the impact to Ansell and to you - our valued partners - was minimal due to our diversified manufacturing approach and the fact that only a small portion of our gloves are produced in China. Additionally, we have recently qualified manufacturing plants outside of China for three of our top Single-Use glove styles, and we continue to qualify alternate plants for other products. These efforts further reinforce our ability to mitigate any tariff-related disruptions and maintain a reliable supply for our customers.
We continue to explore further solutions to reduce the impact of tariffs and improve long-term cost efficiency, and we will continue to keep you informed on these efforts and on the situation as circumstances evolve. In the meantime, should you have any questions or require more information, please do not hesitate to reach out to your local Ansell Territory Manager. We appreciate your continued support and look forward to working together as we navigate these changing circumstances.
Kind Regards,
Sean Sweeney
Chief Commercial OfficerAmericas
Communication Type:
Tarriff
Ansell V#11003
-
03/25/2025
Title: Capsa V#12104
Details:
Tariff Notice
March 17, 2025
Dear Valued Partner,
As a result of increased supply costs attributed to the U.S. tariff policy, Capsa Healthcare will be implementing a price increase effective April 15th, 2025. We are finalizing the price lists and will be distributing within 10 business days of the effective date. During this time of volatile and evolving tariff policy we may need to additionally modify price lists throughout calendar year 2025. During this time, we ask that any price changes be implemented in your systems within 10 business days of notification.
You can be confident that we are making every effort to minimize the impact of the price increases. Thank you for your continued partnership and support while we navigate the uncertain economic conditions and their impact on the global supply chain.
Kindest regards,
Craig Rydingsward
Senior Vice President-Acute
Capsa Healthcare
Communication Type:
Tarriff
Capsa V#12104
-
03/24/2025
Title: Steris V#10863
Details:
Dear Valued Distributor,
STERIS is excited to announce the VERIFY™ RESI-TEST™ SLIDE-THRU Cleaning Process Protein (CPP) Indicator and brushes, the first and only qualitative protein test to receive clearance (Cleared by FDA in November 2024, DEN230085).
The reclassification of residual soil tests like the RESI-TEST indicator is a game changer for healthcare providers, many of which are already using this product.
As a Distributor of impacted SKUs, action is required.
- Update item descriptions and GTINs
- Update website to include new item descriptions, images, and instructions for use (IFU).
- Until further notice, Distributors may only sell items 2D73QH, 2D74QH, and 2D79QH as a complete case.
- Why? Starting March 31, 2025, cleared product will begin shipping.
- VERIFY RESI-TEST SLIDE-THRU Cleaning Process Protein Indicator (LCC101) will include cleared labeling.
- Brush item SKUs 2D73QH, 2D74QH, and 2D79QH labeling will also introduce the cleared product name, VERIFY RESI-TEST SLIDE-THRU Brush on the case label.
- On the outside of each brush case, there will be a letter indicating that brushes can be used as is with the VERIFY RESI-TEST SLIDE-THRU CPP Indicator under the enclosed IFU. Individual brush and box labeling will be updated upon inventory depletion.
Impacted SKUs
SKU
UOM
New Item Description
New GTIN
Other important change
New Image
LCC101
BX
VERIFY RESI-TEST SLIDE-THRU Cleaning Process Protein Indicator
10724995231900
Updated product name, labeling, and expanded viewing box for negative control tests
*Note – item not available to all distributors; distribution status not changing
Steris Verify ResiTest Slide Thru1323 w TMs cmyk.tif | Powered by Box
2D73QH
CA
VERIFY RESI-TEST SLIDE-THRU Brush (1.0-1.2mm)
50724995231915
Product may be sold by CASE only
https://steriscorp.box.com/s/8bhwsu2ebyw5iuxsfu7nwbx6gibka4i4
2D74QH
CA
VERIFY RESI-TEST SLIDE-THRU Brush (1.5-2.6mm)
50724995231922
Product may be sold by CASE only
https://steriscorp.box.com/s/nwftp17z529oix41wax2kgeokied85yt
2D79QH
CA
VERIFY RESI-TEST SLIDE-THRU Brush (2.8-5.0mm)
50724995231939
Product may be sold by CASE only
https://steriscorp.box.com/s/8bhwsu2ebyw5iuxsfu7nwbx6gibka4i4
There are no changes to unit of measures, quantity, or acquisition costs currently.
We appreciate your immediate attention to ensure changes are in place on March 31, 2025.
If you have questions regarding these changes, please contact me at cindy_tanski@steris.com or at 440-445-8508.
Thank you,
Cindy Tanski
Director, Distributor Channel | Healthcare
STERIS
5960 Heisley Road, Mentor, OH 44060
Direct phone: 440-445-8508
Email: cindy_tanski@steris.com
Communication Type:
Product Changes
Steris V#10863
-
03/20/2025
Title: Hollister V#10901
Details:
The notification about the Hollister Pouch Upgrade (HPU) was sent today, wanted to forward along. This will be a 2+ year upgrade process that’ll impact hundreds of Pouch SKU’s between 1-Piece and 2-Piece, with a little less than 100 scheduled to be impacted in 2025; the first SKU’s going through the change will be impacted in May.
No changes to anything from a Dimensions/Weight, UPC, GTIN, HCPCS, UOM perspective, only impacting the physical product within the box.
Thank you as always for the partnership and have a great rest of your week!
Please let me know if you have any questions.
Michelle
Michelle VeNard
Key Account Manager, Channel
2000 Hollister Drive | Libertyville, IL | 60048 USA
c. 404-277-3039
Making a difference in the journey of life
Communication Type:
Product Changes
Hollister V#10901
-
03/19/2025
Title: Stryker Instruments V#10912
Details:
Customer Notification Letter US Version Nasopore
Vendor Item List Sales History 10.21.22-3.19.25
Concordance Team,
Per the attached letter from Stryker, they are voluntarily recalling all lots of Nasopore, Otopore, and Hemopore nasal dressings distributed between 21OCT2022 - 31OCT2024. The letter lists the mfg item numbers, product description, product issue, potential risks and action to be taken. This has been shared with the Concordance Recall Coordinator who will properly handle this with all necessary customers and internal parties.
Communication Type:
Recall Notification
V#10912
-
03/18/2025
Title: Aspen Surgical V#10234
Details:
Please see attached on rebranding of Aspen Surgical L&D items.
What we know
- BRAND CHANGE, The Stork name and logo will be removed from all product descriptions and labels. Individual products tradenames will remain the same (E.g. Amnihook)
- Supplier Change The contract manufacturing site will be changing. Aspen will remain the legal manufacturer.
- Sterilizer Change The contract sterilization site will be changing. The sterilization method will remain the same.
- Country of Origin Change The country of origin for the U-Bag® will be changing from the USA to the Dominican Republic. All other products will remain the same.
I have attached 6 month customer usage and will post all in our Supplier Relations Communications & Notices on our Landing page in Connections.
Please let me know if you have any questions or concerns.
Warmest Regards,
Communication Type:
Product Update
Aspen Surgical V#10235
-
03/18/2025
Title: Nestle V#10183
Details:
Here’s the Nestle inventory letter for this week.
Compleat Organic Blends were removed
Benecalorie was added
Thickenup Clear Packets were added
Compleat Pediatric Peptide 1.0 250 ml was added
Communication Type:
Product Inventory Update
Nestle V#10183
-
03/18/2025
Title: Abbott V#10575
Details:
Abbott is discontinuing the products in the attached letter to prioritize other in-demand options and to streamline our portfolio. The attached customer letter provides details on all of the impacted items.
Estimated out-of-stock dates vary by item based on available inventory and current customer demand. Once inventories are exhausted, no additional quantities will be available. The attached letter contains the list of the products being discontinued, and on page 2, options to consider as an alternative product.
If you have any questions, please contact your Distributor Relations team member from the list below.
Abbott Nutrition Distributor Relations Team Contact Information:
Nick Saffell; Sr. Business Analyst: email: Nicholas.Saffell@abbott.com
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com
Judi Troutman; Manager: email: Judi.Troutman@abbott.com
Thank you,
Joe
Communication Type:
Discontinuation Notice
Abbott V#10575
-
03/18/2025
Title: Capsa V#12104
Details:
Contact your local CAPSA clinical storage consultant
Hello,
My name is Russel Higgins. I am the Director of Sales for Clinical Storage at Capsa Healthcare formerly MASS Medical Storage. I wanted to take a minute to introduce Capsa Healthcare and my team.
Capsa Healthcare is a leading healthcare technology conglomerate known for its point of care computer on wheels, medication management, central fill pharmacy automation and now clinical storage.
I received your email because we either have done business with you in the past or you have shown interest in clinical storage products from MASS Medical Storage.
We produce high quality closed OR storage, medical procedure carts, endoscopy drying and storage solutions. Every product is designed and built to provide you with the best value available in the industry. See our catalog here!
My team of professional storage consultants work with you to design, configure and walk you through the most current storage guidelines for the OR and GI departments. They are also trained to work with project managers, contractors and equipment planners to engineer any project large or small.
If you have a current or future storage project do not hesitate to call or email us. We would be honored to develop a comprehensive proposal to meet your project needs.
Capsa Healthcare Clinical Storage Consultant Contact
Kate Kusch (Northeast) PA, NY, NJ, MD, DC, DE, WV, OH, VT, NH, MA, RI, CT, MA, NH, VT, ME
(480) 679-3378
Ann Lancaster (Southeast) KY, VA, NC, SC, GA, FL, AL, MS, TN
Alancaster@capsahealthcare.com
(704) 897-6537
Spencer LeFort (Southwest) LA, AR, TX, OK, NM, AZ, UT, NV, CA, AZ, HI
(512) 534-1932
Katie Barney (Midwest) MI, IN, IL, MO, IA, MN, WI, ND, SD, NE, KA, CO, WY, MT, ID, WA, OR, AK
(913) 837-6408
Also do not hesitate to call, email or text me if you have any questions.
Sincerely,
Russel Higgins | Director of Sales for the Capsa Storage Division
Capsa Healthcare | Cell: (610) 248-5212
Quick Read: A closer look at ANSI/AAMI St91:2021 for endoscope processing and storage
Communication Type:
Sales Roster
Capsa V#12104
-
03/17/2025
Title: Stryker Instruments V#10912
Details:
Vendor Item List Sales History 9.16.24-3.16.25
Product Updates Bulletin 3.7.25
Concordance Team,
This is from Stryker Instruments:

The sub item 0210-007-000 for obsolete item 0210-008-000 is set up in our system and listed as an equivalent. If there is customer demand for 0210-005-000, please ask the Item Maintenance team if they could set it up.
Communication Type:
Discontinuation Notice
V#10912
-
03/17/2025
Title: Cardiall V#10801
Details:
Rebranding Cardiothoracic letter and customer usage
Rebranding
Cardinal Health is transitioning from Covidien brand and sub-brand names to Cardinal Health brand names. Chest Drainage Units, Thoracic and Trocar Catheters, and Specialty products within our Cardiothoracic product portfolio will be rebranded from Covidien to Cardinal Health brand.Communication Type:
Product Update
Cardinal V#10801
-
03/17/2025
Title: Cardinal V#10801
Details:
Cardinal Glove discontinued letter and Usage
ardinal Health
Item No.
Cardinal Health
Item Description
Alternative Item No.
Alternative Item Description
N8820
Sterile Single Nitrile Exam Glove - S
88SNS02S
Esteem™ Sterile Single Nitrile Exam Glove - S
N8821
Sterile Single Nitrile Exam Glove - M
88SNS03M
Esteem™ Sterile Single Nitrile Exam Glove - M
N8822
Sterile Single Nitrile Exam Glove - L
88SNS04L
Esteem™ Sterile Single Nitrile Exam Glove - L
N8823
Sterile Single Nitrile Exam Glove - XL
88SNS05XL
Esteem™ Sterile Single Nitrile Exam Glove - XL
Communication Type:
Discontinuation Notice
Cardinal V#10801
-
03/17/2025
Title: Midmark V#10978
Details: changes to the Midmark EDI database. The changes include new and discontinued products. The attached EDI file includes the following product updates. There are two tabs in this file:
1. The first tab titled “New Products” contains newly added part numbers and pricing for the Midmark® 631 Procedure Chair available May 6, 2025.
2. The second tab titled “Discontinued Products” contains one discontinued part number, effective November 6, 2025.
Please review your product database and incorporate these changes. Making these changes will facilitate the order process tremendously. If you have questions, please contact Corporate Accounts at: corpaccounts@midmark.com
Designing better care.®
Kurt Forsthoefel
Marketing Director
Medical Division
Midmark CorporationCommunication Type:
Discontinuation Notice
Midmark V#10798
Communication Type:
New Product Launch
Midmark V#10798
-
03/17/2025
Title: Zebra V#13078
Details:
Please see Tariff Price Change Notification below.
Please see links here:
Deal Registration Pricing Policy Partner
List Price Increase Customer Letter- END USER's
On February 20, Zebra communicated a list price adjustment that is going into effect on April 28, 2025. At that time, we referred to the uncertainty of future tariff impacts and noted that any additional list price adjustments with an effective date of April 28 would be communicated separately. Since our original announcement, the following tariffs have been announced:
- The tariff on products from China increased from 10% to 20%.
- A 25% tariff on products from Canada and Mexico went into effect on March 4; however, some exemptions were granted under the USMCA trade agreement until April 2.
As a result of the new tariffs and other cost increases, market conditions and portfolio considerations, an Addendum is being issued that supersedes the previous communication sent on February 20, 2025. This Addendum includes adjustments to select additional products, including those not subjected to tariffs.
Due to the uncertainty of the impacts on products covered under the United States-Mexico-Canada Agreement(USMCA), this new Addendum is segmented into “LIST A” and “LIST B” pricing exhibits.
- LIST A goes into effect on April 28.
- LIST B adjustments will take effect on April 28 unless the pause on tariffs for products covered under the USMCA trade agreement remains in effect through up to April 21. In this case, LIST B price changes will be deferred until further notice. If the tariff rate changes, LIST B price changes may also be adjusted.
For more details, including the products included in List A and B, adjustment percentages and any exclusions, review the notification below.
These changes are being announced early to help minimize disruption to your business and to provide you with sufficient time to react to these changes. The tariff situation remains dynamic, and additional products may become subject to tariffs in the coming weeks or months. Any further list price changes will be communicated separately.
Other Important details:
- Zebra will honor existing pricing to Distributors on open Deal Registration orders placed by Resellers and accepted by Distributors by April 4, 2025, with requested shipment of no later than April 28, 2025, and shipped by June 30, 2025, in accordance with the updated Deal Registration Pricing Policy. Where Zebra list price(s) are increasing, any Deal Registrations containing the impacted products will reflect the new product prices as of the effective date.
- Zebra will honor any previously approved Price Concessions through their expiration date.
If needed, you can download and share the end user price adjustment document as required. We appreciate your continued partnership.
Communication Type:
Tarriff
Zebra V#13078
-
03/16/2025
Title: Kenvue V#10860
Details:
Concordance Team,
Kenvue V#10860 announced that two items are being discontinued in April 2025:

There has been no usage for the past 9 months, so no usage report is attached.
Communication Type:
Discontinuation Notice
V#10860
-
Communication Type:
Discontinuation Notice
Abbott Point of Care V#12125
-
03/12/2025
Title: Nestle V#10183
Details:
Attached is the Nestle Inventory letter for this week
Boost VHC Strawberry was removed
Impact Peptide 1.5 1000 ml was added
Compleat Organic Blends Plant based was added
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Communication Type:
Product Inventory Update
Nestle V#10183
-
03/11/2025
Title: Teleflex V#10538
Details:
Subject: Sterile pre-filled saline syringes will now be packaged and secured on the outside of kits
Please see attachment with Teleflex letter and customer listing with usage and spend for the past 6 months.
If you have any questions please reach out to Heather Burkett. Thanks,
Communication Type:
Product Changes
Teleflex V#10538
-
03/11/2025
Title: BD V#10125
Details:
IV Fluids Update
Please see link with an update on IV Fluids; C#258694 and C#314465.
Please let Heather Burkett know if you have any questions. Thanks,
Communication Type:
Backorder Notification/Update/Reports
BD V#10125
Communication Type:
Allocation Notification/Update
BD V#10125
-
03/11/2025
Title: Medline V#10605
Details:
Medline's Temporary Remedy Ready Care Backorder Notice:
Hello Distributor partners,
We are currently experiencing temporary backorders on the Remedy & Readycare wipe SKUs due to unforeseen manufacturing challenges. The Remedy & ReadyCare division is working to resolve this issue with our manufacturing and inventory management team to get product into branches as soon as possible.
Please see below for all impacted items.
SKUs: MSC099CHG, MSC095311, MSC095107, MSC095106
Item MSC099CHG is no longer a substitute option for item MSC096CHG. The get-well timeframe is currently mid-late April for MSC096CHG & MSC099CHG.

Please see attached letter from Medline. Attached is a customer listing with usage and spend for the past 6 months.
If you have any questions please reach out to Heather Burkett.
Communication Type:
Backorder Notification/Update/Reports
Medline V#10605
-
03/11/2025
Title: BD V#10125
Details:
BD allocation tool for March is attached here.
This also includes a letter for an update on hypo allocations.
Please note: This includes contact info on allocated items and who can approve changes, escalations and increases from each business unit. It also includes a sales roster for each business unit.
Please let Heather Burkett know if you have any questions.
Communication Type:
Allocation Notification/Update
BD V#10125
-
03/11/2025
Title: Abbott V#10575
Details:
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Joe
Joe Corey
Sr. Business Analyst
Abbott Nutrition
Commercial Operations
Abbott
3300 Stelzer Rd.
RP 2-3
Columbus, OH 43219
O:
+1 614-624-6600
F:
+1 614-727-3896
Communication Type:
Product Inventory Update
Abbott V#10575
-
03/9/2025
Title: BD Diagnostics V#10128
Details:
Bactec Update:

Communication Type:
Product Changes
BD Diagnostics V#10128
-
03/8/2025
Title: Health-O-Meter V#10075
Details:
Concordance Team,
Health o meter Professional Scales Announces New ADA Compliant Wheelchair Scale
Pelstar LLC, the manufacturer of Health o meter® Professional Scales, announces the latest addition to its extensive line of wheelchair scales, the new 2620KL Digital Wheelchair Scale with Dual Ramps and Extra-Large Platform. The only wheelchair scale in the market that meets the new MDE Standards for Accessible Medical Diagnostic Equipment. The scale includes critical features that meet ADA safety requirements such as a large 32” x 40” platform, a 2” high platform front edge guard, and dual 1:8 sloped ramps with 2” edge guards. In addition to the new scale, Health o meter® Professional Scales also developed an ADA compliant retrofit kit, available as 2600ADAKIT, for facilities that already utilize 2600KL or 2610KL series scales. The kit provides new components to modify an existing scale and bring it into compliance with the new MDE standards. Utilizing these new scale products not only provide a safer and more comfortable patient weighing experience, they also ensure compliance with ADA rules that mandate facilities utilize equipment that meets certain accessibility standards, which is enforced by the Department of Justice. To learn more visit https://www.homscales.com/ada-mde or call 1-800-815-6615.

Communication Type:
Product Flyer
V#10075
Communication Type:
New Product Launch
V#10075
-
03/7/2025
Title: Ger-Care V#12008
Details:
Geri-Care April 2025 holiday closure announcement
Concordance Team,
Geri-Care v#12008 will be shutdown from April 14th through April 18th for the Passover holiday. In order to accommodate the large influx of orders prior to our closing, orders must be placed on or before April 1st (this has been announced to purchasing and expeditor). They said they will be working overtime to ensure that all orders ship out prior to their shutdown.
Communication Type:
Holiday Schedule
V#12008
-
03/5/2025
Title: Attends V#10055
Details:
Vendor Item List Sales History 2.5.24-3.5.25
Concordance Team,
A 6-month usage file is attached for the following product update from Attends.
PRODUCT UPDATE FROM ATTENDS:
- See two attached flyers (one includes a sizing chart)
- Two years ago, Attends originally converted from the blousy underwear to a more form fitting underwear that did cause some fit issues for our customers.
- Attends is announcing that they previously made some adjustments but there still was a fit issue for some:
- Back bum coverage -- most complaints came from men -The new changes in March address this issue.
- Too tight around the stomach – new changes in March - manufacturing is adjusting top belly area strings to allow it to stretch out further without compromising how the product conforms to the body.
- Attends states that current customers won’t be affected by these changes. The elastics again will still conform to the body per their stretch testing machine.
- First week in March the APV30 (C#191937) will be the first to start shipping out with the changes (also AP030 and APP030 but these are not set up in Concordance system).
- This will slowly roll out for all tiers and sizes throughout March with full conversion by April.
- Once these start shipping, Attends will be targeting accounts to move some customers back to Attends that were lost due to the fit.
- FUTURE PACKAGING CHANGE: Attends is updating the Attends Gender Specific Underwear bags to have real people on them.
- These will start rolling out late March-First of April.
- @Item-Concordance: Tricia asked Attends to email to you the new artwork so you can update the web.

Communication Type:
Product Update
V#10055
-
03/4/2025
Title: Verde EnvironmentalV#12386
Details:
Hi Mary,
Hope you are well. Our marketing team put these together for your sales force.
Can you look them over and give me your stamp of approval before we finalize these?
Deterra_ConcordanceGovernmentDetailer_1.23.25.pdf
Deterra_ConcordanceHealthcareDetailer_1.23.25.pdf
Thanks,
_____________________________
Jake Byrnes| Distributor Sales Manager
Verde Environmental Technologies, Inc.
12900 Whitewater Dr. #200 | Minnetonka, MN 55343
Moblie 218-220-1062
Communication Type:
Product Flyer
Verde Environmental V#12386
-
03/4/2025
Title: Nestle V#10183/NDC V#10030
Details:
Complete retraction notice
Haller, Dillon
WCST
8956
RUSH UNIVERSITY MEDICAL CENTER
4390034927
356615
NUTRITION COMPLEAT 1000ML
CS
36
36
$327.60
Reinhart, Deanna
TIFF
161
WOOD COUNTY HOSPITAL
4390034927
356615
NUTRITION COMPLEAT 1000ML
CS
14
9
$681.32
Jaffe, Joel
WCST
8956
RUSH UNIVERSITY MEDICAL CENTER
4390034927
356615
NUTRITION COMPLEAT 1000ML
CS
2
2
$18.20
Haller, Dillon
KNOX
8939
RUSH UNIVERSITY MEDICAL CENTER
4390034927
356615
NUTRITION COMPLEAT 1000ML
CS
4
4
$218.36
Spellings, Mark
KNOX
891820
ROCKCASTLE REGIONAL HOSPITAL
4390034927
356615
NUTRITION COMPLEAT 1000ML
CS
36
36
$2,034.00
Jaffe, Joel
WCST
8939
RUSH UNIVERSITY MEDICAL CENTER
4390034927
356615
NUTRITION COMPLEAT 1000ML
CS
274
274
$2,493.40
Haller, Dillon
WCST
8939
RUSH UNIVERSITY MEDICAL CENTER
4390034927
356615
NUTRITION COMPLEAT 1000ML
CS
1,563
967
$8,799.64
Good afternoon,
Nestlé Health Science is committed to providing high-quality, science-based nutrition formulas. In the spirit of continuous improvement, please find the attached notice retracting one of the product discontinuations listed in our February 1, 2025 communication.
Please contact your Nestle Distributor Manager with any questions.
Take care,
Amy Sauber, RDN, LDN
Manager, Sales Support Team
Nestlé Health Science U.S.
CONFIDENTIALITY NOTIFICATION: This email message and any attached documents are intended only for the use of the intended recipient(s) and may contain information that is confidential. If you have received this email in error, please notify the above sender and delete it from your computer.
Communication Type:
Product Inventory Update
Nestle V#10183
-
03/4/2025
Title: Baxter V#10797
Details:
Customer Listing Baxter % Increases
Customer Listing_Baxter Alloc Removals
Concordance Team,
The attached Baxter letter indicates the allocation percentage has been increased for some items and removed for some items.
For the affected active items in our system:
- All necessary updates have been made on our side with allocation removals and percentage increases.
- CHS#’s are listed in the Excel file as well as customer listings, one for the removals and one for the increases.
Communication Type:
Allocation Notification/Update
V#10797
-
03/4/2025
Title: J&J V#10196
Details:
Biosurgery Customer Communication_Surgifoam_Supply Disruption_March 2025
Concordance Team,
J&J V#10196 provided a supply disruption update on SURGIFOAM®. They stated due to heightened order volumes of SURGIFOAM®, intermittent shipping delays and notifications regarding backordered products are expected to persist into Q2 2025.
Our inventory analysts marked C#854133 (mfg#1977) as Temp Unav since J&J is cancelling our orders. They ran a usage report and the only customers that came back are below that will need notified.

Communication Type:
Temp Unavailable
V#10196
-
03/3/2025
Title: Medtronic V#10255
Details:
Medtronic Consignee Confirmation Form US_RE00551170_B Appenidx C
Medtronic_Consignee Notification updated with IFU Version (1) US NEW RE00551170_C
List of Affected Products - Attachment A
Vendor Item List Sales History 8.26.24-2.25.25
Concordance Team,
Our recall team has addressed this supplier communication.
- As the vendor states below, there is a confirmation form to be completed.
- The Excel file “vendor item sales history” is the Concordance usage report that shows affected customers and account managers (past 6 months).
MESSAGE FROM MEDTRONIC:
Urgent Medical Device Correction
Medtronic is issuing an update to the Instructions for Use (IFU) for Microstream™ Advance Neonatal-Infant and Adult-Pediatric Intubated CO2 Filter Line, Microstream™ Luer Adult-Pediatric Intubated CO2 Filter Line and Microstream™ Luer Adult-Pediatric Airway Adapter Sampling Line devices.
Actions you should take:
- Continue using the Microstream™ Advance Neonatal-Infant and Adult Pediatric Intubated CO2 Filter Line, Microstream™ Luer Adult-Pediatric Intubated CO2 Filter Line and Microstream™ Luer Adult-Pediatric Airway Adapter Sampling Line devices.
- Pass on and post this notice for all those who need to be aware within your organization and to any organization where the affected product has been transferred or distributed.
- Please complete and return the enclosed Customer Confirmation Form to rs.gmbmitgfca@medtronic.com even if you do not have unused inventory.
Medtronic Division: Med Surg / Acute Care & Monitoring (Respiratory)
If you have any questions regarding this communication, please contact your Medtronic Representative or Customer Service at 800-962-9888, Option 2.
Communication Type:
Updated Supplier Information
V#10255
-
03/3/2025
Title: Health-o-Meter V#10075
Details:
Concordance Team,
ANNOUNCEMENT FROM HEALTH-O-METER V#10075:
Below is an announcement on our new ADA Compliant Wheelchair Scale to send out to the sales team.
Health o meter Professional Scales Announces New ADA Compliant Wheelchair Scale
Pelstar LLC, the manufacturer of Health o meter® Professional Scales, announces the latest addition to its extensive line of wheelchair scales, the new 2620KL Digital Wheelchair Scale with Dual Ramps and Extra-Large Platform. The only wheelchair scale in the market that meets the new MDE Standards for Accessible Medical Diagnostic Equipment.
The scale includes critical features that meet ADA safety requirements such as:
- Large 32” x 40” platform
- 2” edge protection on platform
- 2” edge protection on ramps
- 1:8 sloped ramps
In addition to the new scale, Health o meter® Professional Scales also developed an ADA compliant retrofit kit, available as 2600ADAKIT, for facilities that already utilize 2600KL and 2610KL wheelchair scales. The kit provides new components to modify an existing scale and bring it into compliance with the new MDE standards. Utilizing these new scale products not only provide a safer and more comfortable patient weighing experience, they also ensure compliance with ADA rules that mandate facilities utilize equipment that meets certain accessibility standards, which is enforced by the Department of Justice.
Click here to learn more about the 2620KL and ADA compliance, or reach out to Brett Lucido, Director of National Accounts, blucido@homscales.com, (724) 799-4233 if you have any questions or would like more information.
Communication Type:
New Product Launch
V#10075
-
03/3/2025
Title: Health-o-Meter V#10075
Details:
Concordance Team,
NEW PRODUCT ANNOUNCEMENT FROM HEALTH-O-METER V#10075:
Health o meter Professional Scales Announces New ADA Compliant Wheelchair Scale
Pelstar LLC, the manufacturer of Health o meter® Professional Scales, announces the latest addition to its extensive line of wheelchair scales, the new 2620KL Digital Wheelchair Scale with Dual Ramps and Extra-Large Platform. The only wheelchair scale in the market that meets the new MDE Standards for Accessible Medical Diagnostic Equipment. The scale includes critical features that meet ADA safety requirements such as a large 32” x 40” platform, a 2” high platform front edge guard, and dual 1:8 sloped ramps with 2” edge guards. In addition to the new scale, Health o meter® Professional Scales also developed an ADA compliant retrofit kit, available as 2600ADAKIT, for facilities that already utilize 2600KL or 2610KL series scales. The kit provides new components to modify an existing scale and bring it into compliance with the new MDE standards. Utilizing these new scale products not only provide a safer and more comfortable patient weighing experience, they also ensure compliance with ADA rules that mandate facilities utilize equipment that meets certain accessibility standards, which is enforced by the Department of Justice. To learn more visit https://www.homscales.com/ada-mde or call 1-800-815-6615.
SKU
Description
Cost
MSRP
2620KL
Digital Wheelchair Scale with Dual Ramps and Extra-Large Platform, ADA Compliant
$2723
$4473
2620KG
Digital Wheelchair Scale with Dual Ramps and Extra-Large Platform, ADA Compliant, KG only
$2723
$4473
2600ADAKIT
ADA Compliance Kit For 2600 and 2610 Series Scales
$495
$814
Communication Type:
New Product Launch
V#10075
-
03/2/2025
Title: Solventum V#10296
Details:
3M™ Attest™ eBowie-Dick Test System - Final Nov. 2024
3M™ Attest™ eBowie-Dick NPI Data Packet
Concordance Team,
There was a previous post in November 2024 about new productS from Solventum per the attachments. The availability was original slated for January 1, 2025, but it was delayed. The supplier is announcing that it is available effective March 1, 2025. If you have customer demand for the new product, reach out to our Item Maintenance team to request item set up.
Communication Type:
New Product Launch
V#10296
-
03/2/2025
Title: Solventum V#10296
Details:
3M™ Attest™ Super Rapid Vaporized Hydrogen Peroxide Clear Challenge Pack– Final Feb. 2025
3M™ Attest™ Super Rapid Vaporized Hydrogen Peroxide Clear Challenge Pack NPI Data Packet
Concordance Team,
Solventum V#10296 is launching a new product. Please see the attached sell sheet and letter which includes this message:

Please let the Item Maintenance team know if you need the items set up in our system due to new customer demand.
Communication Type:
New Product Launch
V#10296
-
03/1/2025
Title: Attends V#10055
Details:
Communication Type:
Product Changes
Communication Type:
Product Flyer
-
02/28/2025
Title: Airlife V#11179 - Sales Roster Update
Details:
AirLife US Acute Sales Roster 12.4.24
Concordance Team,
Attached is the most up up to date sales roster from Airlife V#11179.
With any questions, please reach out to Jeff Shook.
Kind Regards
Communication Type:
Sales Roster
Airlife V#11179
-
02/28/2025
Title: Airlife V#11179 - Recall Notice
Details:
Smiths Medical ETT Recall Notice
Concordance Team,
Be advised that:
On February 19th, 2025, Smiths Medical issued a notification regarding the recall of pediatric size 2.5, 3.0 and 3.5 Uncuffed Oral/Nasal Endotracheal Tubes.
With any further questions, please reach out to Jeff Shook.
Kind Regards
Communication Type:
Recall Notification
Airelife V#11179
-
02/28/2025
Title: Coloplast V#10581
Details:
Sea-Clens Woun' Dres Discontinuation Letter
Vendor Item List Sales History 8.28.24-2.28.25
Concordance Team,
The attached PDF letter from Coloplast V#10581 is a notification about four items that will be discontinued by May 31, 2025. Each of the four items have subs from other manufacturers loaded in VAI. Our 6-month usage file is also attached.
Communication Type:
Discontinuation Notice
V#10581
-
02/27/2025
Title: BSN V#10231
Details:
Jobst Discontinuation and Product launch with BSN Medical.
Please see customer listing with usage here. Please see official notification from BSN here.
Product Announcement January 2025 JOBST® Anti-Em/GP C# REF# Style Size/Length UOM Availability REF# Style Size/Length UOM Availability 746016 111402 Knee SM/Reg 12 Until 04/30/25 7702600 Knee SM/Reg 10 05/01/2025 746032 111406 Knee MD/Reg 12 Until 04/30/25 7702700 Knee MD/Reg 10 05/01/2025 746057 111410 Knee LG/Reg 12 Until 04/30/25 7702800 Knee LG/Reg 10 05/01/2025 746073 111414 Knee XL/Reg 12 Until 04/30/25 7712400 Knee XL/Reg 10 05/01/2025 7713300 Knee 2XL/Reg NEW Size 10 05/01/2025 111403 Knee SM/Long 12 Until 04/30/25 7702610 Knee SM/Long 10 05/01/2025 746040 111407 Knee MD/Long 12 Until 04/30/25 7702710 Knee MD/Long 10 05/01/2025 746065 111411 Knee LG/Long 12 Until 04/30/25 7702810 Knee LG/Long 10 05/01/2025 746081 111415 Knee XL/Long 12 Until 04/30/25 7712410 Knee XL/Long 10 05/01/2025 7713310 Knee 2XL/Long NEW Size 10 05/01/2025 746099 111450 Thigh SM/Short 6 Until 04/30/25 Not available 746107 111451 Thigh SM/Reg 6 Until 04/30/25 4633500 Thigh SM/Reg 10 05/01/2025 875799 111452 Thigh SM/Long 6 Until 04/30/25 4633600 Thigh SM/Long 10 05/01/2025 746123 111454 Thigh MD/Short 6 Until 04/30/25 Not available 746131 111455 Thigh MD/Reg 6 Until 04/30/25 4633700 Thigh MD/Reg 10 05/01/2025 746149 111456 Thigh MD/Long 6 Until 04/30/25 4633800 Thigh MD/Long 10 05/01/2025 746156 111458 Thigh LG/Short 6 Until 04/30/25 Not available 746164 111459 Thigh LG/Reg 6 Until 04/30/25 4638800 Thigh LG/Reg 10 05/01/2025 746172 111460 Thigh LG/Long 6 Until 04/30/25 4638900 Thigh LG/Long 10 05/01/2025 746180 111462 Thigh XL/Reg 6 Until 04/30/25 7712500 Thigh XL/Reg 10 05/01/2025 746198 111463 Thigh XL/Long 6 Until 04/30/25 7712800 Thigh XL/Long 10 05/01/2025 949024 111464 Thigh 2XL/Reg 6 Until 04/30/25 7713000 Thigh 2XL/Reg 10 05/01/2025 118042 111465 Thigh 2XL/Long 6 Until 04/30/25 7713100 Thigh 2XL/Long 10 05/01/2025 746206 111625 Waist High SM/Reg 6 While Supplies Last 7961102 Double Chap SM/Reg 5 05/01/2025 746214 111626 Waist High SM/Long 6 While Supplies Last 7961202 Double Chap SM/Long 5 05/01/2025 746222 111627 Waist High MD/Reg 6 Discontinued 8/12/2021 7961302 Double Chap MD/Reg 5 05/01/2025 746230 111628 Waist High MD/Long 6 While Supplies Last 7961402 Double Chap MD/Long 5 05/01/2025 746248 111629 Waist High LG/Reg 6 While Supplies Last 7961502 Double Chap LG/Reg 5 05/01/2025 941781 111630 Waist High LG/Long 6 While Supplies Last 7961602 Double Chap LG/Long 5 05/01/2025 Communication Type:
Discontinuation Notice
BSN V#10231
Communication Type:
New Product Launch
BSN V#10231
-
02/27/2025
Title: Abbott V#10575
Details:
Abbott weekly Update
We are sharing with all of our distributor partners the attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Joe
Joe Corey
Sr. Business Analyst
Abbott Nutrition
Commercial Operations
Abbott
3300 Stelzer Rd.
RP 2-3
Columbus, OH 43219
O:
+1 614-624-6600
F:
+1 614-727-3896
This communication may contain information that is proprietary, confidential, or exempt from disclosure. If you are not the intended recipient, please note that any other dissemination, distribution, use or copying of this communication is strictly prohibited. Anyone who receives this message in error should notify the sender immediately by telephone or by return e-mail and delete it from their computer.
Communication Type:
Product Inventory Update
Abbott V#10575
-
02/27/2025
Title: Ansell V#11003
Details: Gammes usage color change
Ansell
Email not displaying correctly? View it in your browser.
Ansell Healthcare Products, LLC
----
111 Wood Avenue South
Iselin, NJ 08830
United States
T. + 1 732 345 5400
F. + 1 732 219 5114
www.ansell.comFebruary 7, 2025
Dear Valued Ansell Customer,
At Ansell, we are constantly assessing customer feedback and evaluating our product portfolio to ensure we have the right products to meet your needs. Accordingly, as part of our long-term commitment to providing the most clinically driven products for today’s healthcare professionals, we are happy to announce the color enhancement of our GAMMEX® Non-Latex PI Green surgical gloves. These gloves will now feature a darker shade of green, allowing for increased versatility and enabling the gloves to be used throughout your entire facility. The darker green color is ideal for use as underglove in double gloving situations, supporting best practice guidelines from CDC, AORN, and other health organization. GAMMEX Non-Latex PI Green can also continue to be used for all surgeries as a dedicated stand-alone glove.
Please find below a visual comparison of the old vs. new color enhacement:The updated glove color for the GAMMEX® Non-Latex PI Green will be introduced into the market late February 2025. Please note that during this transition, you may briefly see a mixture of both current and new color gloves as the older product filters through our distribution centers. To minimize any disruption in the transition process, there will be no change to the ordering codes. Additionally, the quality, performance and specs of the gloves remain unchanged.
For your convenience, please refer to the product datasheet for detailed information on the GAMMEX® Non-Latex PI Green surgical glove. For additional information, please visit www.ansell.com or contact your Ansell Medical Sales Representative or Ansell Customer Service at 1-800-952-9916.
Thank you for your support of Ansell. We look forward to our continued partnership in providing premium safety solutions.
Sincerely,
Andrew Hurdle
Sr. Marketing Manager – Acute Care
Medical – North AmericaAnsell, ® and ™ are trademarks owned by Ansell Limited or one of its affiliates, except as noted.
© 2025 Ansell Limited. All Rights Reserved.Communication Type:
Product Update
Ansell V#11003
Communication Type:
Product Changes
Ansell V#11003
-
02/26/2025
Title: Essity V#10272
Details:
TENA Discontinuation Update:
Please see original notice on here. Customer Usage for the past 6 months is here.
The following items have been discontinued with suggested subs:
S#287164 #54283 disc and suggested sub S#241417 #62326S#252079 #54427 disc and suggested sub S#243338 #47709
S#287181 #54268 disc and suggested sub S#234539 #47619
S#515569 #351 disc and suggested sub S#166113 #350
S#516096 #354 disc and suggested sub S#515940 #353
S#103419 #67837 disc and suggested sub S#140605 #67902
S#103420 #67838 disc and suggested sub S#140607 #67903
S#160547 #67804 disc and suggested sub S#226012 #61199
S#160546 #67805 disc and suggested sub S#131819 #67802
S#160548 #67806 disc and suggested sub S#131820 #67803
S#160550 #67807 disc and suggested sub S#131820 #67803
Communication Type:
Discontinuation Notice
Essity V#10272
-
02/25/2025
Title: Amsino V#10385
Details:
Navigating New Tariffs: Amsino's Plan
Please see letter from Amsino below.
Dear Jeff,
As you may know, the incoming US Administration has introduced tariffs on goods manufactured outside of the US. While we are excited about expanding our onshore and nearshoring operations, many Amsino products are still manufactured in our China facilities, which means we are facing the immediate impact of these tariffs.
We are currently exploring options for how we will respond to these tariffs and will share additional updates as we reach a conclusion on how to move forward. Your dedicated Amsino representative will be reaching out to you shortly to provide any additional details and answer any questions you may have.
We value your continued partnership as we navigate these ongoing changes.
Jeff Reid
President, North America
Amsino International, Inc.
Communication Type:
Tarriff
Amsino V#10385
-
02/25/2025
Title: BD V#10125
Details:
Updated 2025 Holiday Schedule

Communication Type:
Holiday Schedule
BD V#10125
-
02/25/2025
Title: Medline V#10605
Details:
Oral Care Kits - Inventory Delays
Distributor partners,
As you may be aware, we are currently experiencing backorders on some of our Oral Care Kits due to manufacturing delays. Medline is working to resolve these issues and get product into branches as soon as possible.

We apologize for any inconvenience this has caused, and we are committed to help mitigate this disruption.
Communication Type:
Backorder Notification/Update/Reports
Medline V#10605
-
02/24/2025
Title: Holliter V#10901
Details:
FormaFlex Kit Discontinuation Letter
Dear Valued Customer,
Please see the attached important communication regarding the Hollister Ostomy product portfolio.
As always, please reach out to your Hollister Account Manager or Customer Service at 800.323.4060 if you have any questions.
Thank you,
Nico Volpe
Senior Marketing Coordinator, US Ostomy / Continece Care Marketing
2000 Hollister Drive | Libertyville, IL | 60048 | US
o: +1.847.932.1106 | m: +1.224.358.2222
Making a difference in the journey of life
Communication Type:
Discontinuation Notice
V#10901
-
02/24/2025
Title: Hollister V#10901
Details:
Hollister Allocation customer usage
Effective this Monday Feb 10, the following AnchorFast SKU’s that were affected by the voluntary recall, are available for purchase in the allocated quantities across the Concordance network.
SKU
Status
Weekly Allocation QTY across OM Network
9799
Available for Purchase since 1/27/25
75 BX
9800
Available for Purchase 2/10/25
17 BX
9787
Available for Purchase 2/10/25
Not on allocation / no threshold
9700
Available for Purchase 2/10/25
Not on allocation / no threshold
If it’s possible for the next few weeks to continue placing these AnchorFast PO’s separate from other Hollister product, that would significantly relieve stress on our team(s) prioritizing fulfillment of these orders. Any PO’s received in excess of the allocated quantities will be deleted.
Let me know if you have any questions,
Michelle
Michelle VeNard
Key Account Manager, Channel
2000 Hollister Drive | Libertyville, IL | 60048 USA
c. 404-277-3039
Making a difference in the journey of life
Communication Type:
Allocation Notification/Update
Hollister V#10901
-
02/19/2025
Title: ICU V#10917
Details:
ICU IV Solutions- Updated Allocation/ Direct ONLY Ordering
Please see attached letter from ICU and our Customer Listing with Usage.
SUBJECT: Updated Allocation Actions for Select Sodium Chloride Small Volume Presentations
Dear Valued Customer,
ICU Medical is continuing to experience unprecedented demand, within our contracted, committed customer base, for our 50 mL and
100 mL 0.9% Sodium Chloride Injection, USP product presentations due to the ongoing and dynamic IV Solution supply market
conditions.
Effective March 1, 2025, we will transition the enclosed Sodium Chloride product presentations to Direct Purchase Only for
contracted, committed customers with a purchase history from October – December 2024. All unfulfilled and attempted purchase
orders placed after this date will be cancelled for uncommitted customers. This applies to both direct orders and orders placed through
trading partners.
If you are a contracted, committed customer, please continue to order through your trading partner network through the month of
February or until the respective inventory is depleted. All direct committed customer allocations at the time of this letter’s distribution will
remain unchanged. Customers should be aware that in order for ICU Medical to maintain the current 105% allocation level, effective
immediately until market conditions resolve, ICU Medical may fulfill orders with 4:1 (quad) or 1:1 (single) product
configurations interchangeably to allow our contracted, committed customers to receive their total single and quad small
volume bag monthly allocation.
We are working dilgently to continue to increase production to meet the immediate and future needs of our contracted and committed
customers, and will continue to provide updated supply information as it becomes available.
Allocated Products for Direct, ICU Medical Committed Only Customer Purchases:
Communication Type:
Allocation Notification/Update
V#10917
-
02/18/2025
Title: GOJO V#10354
Details:
FROM GOJO V#10354: We are excited to announce that our PURELL® Surface Multi-Purpose Disinfecting Wipes, and their associated dispensers, are ready to ship! These high-capacity wipes kill 99.9% of germs with the EPA’s lowest toxicity rating. Also launching is our PURELL® Thermometer Probe Wipes - Convenient wipes made with food-contact safe ingredients that quickly and effectively remove food soils on probes.
- 9103-02 – PURELL® Surface Multi-Purpose Disinfecting (High-Capacity) Wipes, 2 - 1200 Count Refills
- 9117-01-SLVSFWP – PURELL® Surface DS360 Stand Dispenser
- 9103-01 – PURELL® Surface High-Capacity Wall Mount
- 9211-1M - PURELL® Thermometer Probe Wipes, 1000 Individually Wrapped
Communication Type:
Product Flyer
V#10354
-
02/17/2025
Title: Abbott V#10575
Details:
Nutrition Inventory Letter/update
We are sharing with all of our distributor partners the attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Joe
Joe Corey
Sr. Business Analyst
Abbott Nutrition
Commercial Operations
Abbott
3300 Stelzer Rd.
RP 2-3
Columbus, OH 43219
O:
+1 614-624-6600
F:
+1 614-727-3896
Communication Type:
Product Inventory Update
V#10575
-
02/17/2025
Title: Kate Farms V#12409
Details:
Kate Farms New Product Launch Letter
We are excited to announce the expansion of the Kate Farms® pediatric portfolio with four new products:
Pediatric Peptide 1.0 in Strawberry and Kids Nutrition in Vanilla, Chocolate, and Strawberry flavors.
At Kate Farms, our mission is to make nutrition the foundation of human well-being. Our innovative,
plant-based, organic products are designed to provide high-quality nutrition and are made without
common allergens or artificial sweeteners, flavors, colors, or preservatives — making them suitable for a
wide range of patients.
Our pediatric portfolio is designed to help support children’s growth and development and is eligible for
insurance coverage. The new Pediatric Peptide 1.0 in Strawberry is one of our most requested products,
offering another great-tasting peptide formula for tube feeding and oral use. Kids Nutrition is available in
three delicious flavors for oral nutrition supplementers, may also be used for tube feeding, and contains
DHA from a plant-based source.
Available
February
Available
March
Product
Kate Farms®
Pediatric Peptide 1.0
Strawberry
Kate Farms®
Kids Nutrition
Vanilla, Chocolate, and Strawberry
Availability February 18, 2025 March 4, 2025
Packaging 12 - 250 mL cartons/case 12 - 250 mL cartons/case
Calories/Carton 250 Cal/250 mL 250 Cal/250 mL
HCPCS Code B4161 B4160
UPC Carton 811112031314 811112031192 811112031215 811112031239
UPC Case 811112031321 811112031208 811112031222 811112031246
NDC Format # 11112-0031-31 11112-0031-19 11112-0031-21 11112-0031-23
Kate Farms formulas stand out by being plant-based, made for tolerance, and containing a unique fiber
blend that helps support the microbiome and gut health.1,2 We use high-quality, clean† ingredients, free
from artificial sweeteners, flavors, colors, or preservatives. Our formulas are in stock and available.
Kate Farms is carried by major distributors. Additional product information for Pediatric Peptide 1.0 in Strawberry
and Kids Nutrition in Vanilla, Chocolate, and Strawberry flavors can be found on the next page. Contact your
Kate Farms representative to learn more.
All Good Things®
* Independent plant-based formula survey.
† Clean: Certified USDA Organic, Non-GMO Project Verified, and Gluten-Free Certified; no artificial sweeteners, and no artificial flavors, colors, or preservatives; no coCommunication Type:
New Product Launch
V#12409
-
Communication Type:
Product Inventory Update
V#10183
-
02/17/2025
Title: Baxter V#10797
Details:
Concordance Team,
The inventory analyst team has increased the allocation to 110% on the following SKUs after receiving the attached allocation update from Baxter V#10797.
- This will reflect on the customers allocation report tomorrow morning, 2/18/25.
- Customer listing is attached.
MFG# CHS #
2B1324X 404483
2B0063Q 543223
2B0062Q 604967
2B2323Q 792838
2B2322Q 246819
2B7127 605261
2B7487 954685
Communication Type:
Allocation Notification/Update
V#10797
-
02/17/2025
Title: Cardinal V#10801
Details:
Cardinal is excited to launch a 3 new product to add to their portfolio
- Biodegradable Exam Glove for your customers with green initiatives.
- Stretch Brief to add to their incontinence line.
- Smartflow Compression System
- Attached are sales flyers for both along with spreadsheet with corresponding CHS’s #’s
- All information will be posted in our Supplier Relations Communications & Notices landing page on Connections
As a reminder, we have a growth incentive with Cardinal for 2025 on Incontinence and Compression products.
Please reach out to me with any questions or additional needs.
Communication Type:
New Product Launch
V#10801
-
02/15/2025
Title: Welch Allyn V#10834
Details:
Vendor Item List Sales History 8.14.24-2.14.25
Concordance Team,
Welch Allyn has discontinued mfg#4302 (C#298123) and mfg#5125XX (C#270880)and 5125KX (C#284368). The attached usage file reveals only #284368 has been sold in the past 6 months.
Communication Type:
Discontinuation Notice
V#10834
-
02/14/2025
Title: BD V#10125
Details:
Updated Allocation Tool for February is attached. This includes additional items that have been added since the first file for February was sent.
If you have any questions, please reach out to Heather Burkett. Thanks,
Communication Type:
Allocation Notification/Update
V#10125
-
02/13/2025
Title: Medline V#10605
Details:
UOM Change
We are notifying you of an upcoming update to the Unit of Measure (UoM) to more accurately reflect the physical count of packets rather than the number of swabsticks. This change is currently in progress and will go live soon.
MDS093902 is our triple-packed PVP swabstick, meaning each packet contains three swabs. Currently, the UoM in our database reflects the total number of swabsticks, rather than the total number of physical packets. This update will change the UoM in our database to reflect the number of packets, reducing any future confusion and allowing us to more accurately track our quantities. This change will likely impact your account's internal item code/UoM and how it is recorded in your records. Please note that nothing about the product, pricing, shipping, or packaging will change, just our internal database.
SKU (UM): Current UoM --> New UoM
MDS093902 (Case): 750/CS --> 250/CS
MDS093902ZZ (Box): 75/BX --> 25/BX
MDS093902H (Each): 3/PK --> 1 PK, 3/PK
Please reach out if you have any questions regarding this update. The UoMs of MDS093902 will be identical to those of MDS093810, our triple-packed Alcohol Swabsticks.
Communication Type:
Product Changes
V#10605
Communication Type:
Product Update
V#10605
-
02/13/2025
Title: MIdmark V#10987
Details:
Technical Bulletin
Date: January 28, 2025
To: Distribution Partners
From: Mark Hoying, Manager, Senior Post Sales Support
Topic: Medical—Parts Discontinuation NoticeDear Distribution Partners:
At Midmark, we take pride in providing our customers with quality products and services that can be relied upon to improve efficiency and patient care while ensuring integrity, performance and safety. For these reasons, Midmark has made the decision to end the product service for several models that have been out of production for more than ten (10) years. Acquiring parts for these older models from Midmark authorized suppliers has become limited, leaving us unable to service the products and our customers effectively.
Effective immediately, parts for the product models listed below are no longer available from Midmark. The affected models include the following:NCO011039
End of Service for 622-007, 622-008 and 623-007 Chairs (Items unique to these Products will be made Obsolete.)
NCO011046
End of Service for 250-001 Lights
NCO011051
End of Service for 255-008 Lights
NCO011053
End of Service for M9D-020 Sterilizer
For questions, please contact Medical Technical Support at 1.844.856.1230.
Designing better care.®
Mark Hoying
Manager, Senior Post Sales Support
Midmark CorporationCommunication Type:
Updated Supplier Information
V#10987
-
02/11/2025
Title: J&J V#10196
Details:
GUT Return to Service (Distributor) - 1915G, U202H, K895H
Concordance Team,
Per J&J V#10196, mfg#1915G (C#515015) is now available for us to purchase again. Our inventory analyst team has removed it from temp unavailable status and provided the attached customer listing. It is advised that you check the stock requests and update if needed.
Communication Type:
Temp Unavailable
V#10196
-
02/10/2025
Title: ICU V#10917
Details:
Joint Venture Notification :
Please reach out to Heather Burkett if you have any questions. Thanks,

Communication Type:
Updated Supplier Information
V#10917
-
02/10/2025
Title: Essity V#10272
Details:
Cutimed Sorbact Swab Discontinuation. Please see attached on C#323942. This isn’t currently stocked in any locations. Please reach out to Heather Burkett if you have any questions.

Communication Type:
Discontinuation Notice
V#10272
-
02/10/2025
Title: ICU V#10917
Details:
BCI Patient Monitoring Discontinuation: Please see from ICU Medical. None of these items are currently set up in our system. Please reach out to Heather Burkett if you have any questions. Thanks,

Part Num Description Inventory as of Jan 31 2025 1105 ADAPTER, ARWY STRAIGHT W/FLT 10/PK - 1123 SAMPLE LN ORL/NAS CO2 ADT PK10 10/PKG 220 1128 NAFION TUB 2FT EXTENSION 10/PKG 10 1151 PEDI AIRWY ADPT W/O FLTR PK10 10/PKG 1,390 1152 ADAPTER AIRWAY W/FILTER 10PKG 1/EA - 1179 ABSORBER CO2 9004 2/BX 196 1300 DISP OXIMETRY PROBE ADULT BX10 10/BX 740 1301 PROBE OXIMETRY DISPOSABLE PEDIATRIC 10/BX 6,830 1302 DISP OXIMETRY PRO.NEONATE BX10 10/BX 4,595 1303 DISP OXIMETRY PROBE-INFNT BX10 10/BX 20,600 1502 GAS, CALIBRATION, CYLINDER, 10% CO2, 50% N2O, 1.5% ISOFLURANE, BAL N2, 9L 1/EA - 1613 AC/DC POWER SUPPLY 100-240 VAC / 12VDC (US, JAPAN) 1/EA 53 1616 CHARGER AC/POWER SUPPLY 100-240VAC/24VDC (US, JAPAN) 125 3043 OXIMETRY PROBE UNIVERSAL Y 1/EA 535 3049 STRIPS ADHESIVE 20/PKG 40 3311 CABLE OXIMETRY 5FT BCI 1/EA 395 3315 HAND HELD CARRY CASE 1/EA 166 3387 ROLL STAND KIT REDESIGN GCX ADVISOR 1/EA 29 3393 KIT WALL MOUNT GCX REDESIGN 9200 1/EA 22 3409 PAPER PRINTER 3408/HP PRINTER REF 8411 ROLLS 4/BX 136 3444 SENSOR OXIMETRY FINGER PROBE COMFORT CLIP 1/EA 1,799 5093 GAS, CALIBRATION, CYLINDER, 10% CO2, 21% O2, BAL N2, 19L 1/EA 71 6012 PAPER PRINTER 3401/6004 4/PKG 32 6014 TEMP PROBE COVER F/6004 BX1000 1,000/BX 18,000 6111 PRINTER PAPER FOR 6100 4/BX 1,788 8044 SAMPLE LINE 8 FT 10/PKG 4,180 8061 CALIBRATED REGULATOR-GAS FLOW 1/EA 43 8075 MOISTURE TRAP W/FEMALE ADAPTER 1/EA 2,009 8133 REGULATOR, CAL. GAS FLOW 1/EA - 8208 GAS MANIFOLD FILTERS 10/BG ROW 1,430 8211 SAMPLE LINE, 4FT 10/PKG 780 8216 CHARGER AC 100V 50HZ 1/EA - 8217 8200 CALIB.KT(5093,8061,8211,8223) 1/EA 22 8223 CALIBRATION ADAPTER 1/EA - 8404 BATTERY CHARGER FOR 8400 1/EA 41 8408 BATTERY (LITHIUM ION) 8400 1/EA 16 8413 COMPLETE CASE FOR 8400 1/EA - 9014 9004 3-CHAN ANALOGUE CABLE 1/EA - 9048 FILTERS FOR 9004 2/PKG 4,210 9189 ADAPTER "T" 10/PK 450 1101A CANNULA NASAL ADULT 10/PKG 4,860 1102P CANNULA NASAL PEDIATRIC 10/PKG 220 21-0468-01 POWER SUPPLY, 100-240VAC, 50/60Hz, 3404 1/EA - 3044S ADULT SPOT-CHECK SENSOR (WW1000, WW1020, WW1030) 1/EA 1,142 3311L HAND-HELD OXIMETRY CABLE 15FT 1/EA 47 3312R CASE REPLACEMENT BCI 3301 REDESIGN 1/EA 4 70-0321 PWB ASM DIGITAL OXI BRD OEM, VNX4B 1.30 1/EA 10 71552B1 SpO2 CONTROLLER "BC" 16 VX010 VIOMEDEX RESPIRATION SENSOR 50/BX 6,550 WW1000EN-00 MONITOR H-H SPECTRO2 WW1000 SYSTEM ENGLISH, LOANER 1/EA - WW1000P1EN MONITOR H-H SPECTRO2 WW1000 SYSTEM W/3178 PED SENSOR ENGLISH 1/EA - WW1020A1EN MONITOR H-H SPECTRO2 WW1020 W/3044 SYSTEM ENGLISH 1/EA 16 WW1020A1EN-00 MONITOR WW1020 SYSTEM W/3044 ENGLISH, LOANER 1/EA - WW1020EN MONITOR H-H SPECTRO2 WW1020 SYSTEM ENGLISH 1/EA 62 WW1020EN-00 MONITOR H-H SPECTRO2 WW1020 SYSTEM ENGLISH, LOANER 1/EA - WW1030BFR MONITOR H-H SPECTRO2 WW1030 SYSTEM W/ WW1018B GLOVE FRENCH 1/EA - WW1030EN MONITOR H-H SPECTRO2 WW1030 SYSTEM ENGLISH 1/EA 86 WW1030EN-00 MONITOR H-H SPECTRO2 WW1030 SYSTEM ENGLISH, LOANER 1/EA - WW1080 KIT SENSOR CRADLE SPECTRO2 1/EA 1,324 WW1095 POWER SUPPLY UNIVERSAL WITH US/JAPAN ADAPTER 30WATT 1/EA 310 WW1095OUS POWER SUPPLY UNIVERSAL WITH INTERNATIONAL BLADES 30WATT 1/EA 373 WW1100 ADAPTER AIRWAY 12.7mm I.D. X 15mm O.D. STRAIGHT FILTERLESS 10/PKG - WW1129 SAMPLE LINE NASAL CO2 ADULT REF SALTER LABS 4000 10/PKG 2,040 WW1140 SAMPLE LINE EXTENSION 15FT 10/PKG 967 WW3078 OXIMETRY EAR PROBE 1/EA 3,871 WW3711 PACKAGED PWA DIGITAL MICRO POWER OXIMETRY OEM 1/EA 4,526 WW8030 CAPNOCHECK ELBOW 10/PKG 790 WW8214 ACCESS. KIT-1100, 8211, 8208 10/PKG 477 Communication Type:
Discontinuation Notice
V#10917
-
02/10/2025
Title: Phase Scientific V#12840
Details:
We’re excited to bring you a special promo on some of our most essential diagnostic products. From flu season to everyday diagnostic needs, we’ve got you covered!
How It Works:
Purchase a combined total of $5,000 worth of the following products:
- SpeedySwab COVID-19 + Flu A&B Tests (OTC & POC)
- INDICAID COVID-19 Rapid Antigen Tests (OTC & POC)
- INDICAID Rapid Strep A Antigen Test (POC)
Get (1) case of your choice from the following products as a bonus:
- INDICAID iFOB Fecal Occult Blood Tests (OTC & POC)
- INDICAID UTI Rapid Test Strip (OTC)
- INDICAID Ovulation Test Strips (OTC)
- INDICAID Multi-Drug Urine Test Cups (POC)
- INDICAID Fentanyl Urine Detection Test (POC)
Communication Type:
End User Promo
V#12840
-
02/10/2025
Title: BD V#10125
Details:
Allocation tool for February for BD Medical.
Please see above link for BD's updated allocation tool for February 2025.
Please note: This includes contact info on allocated items and who can approve changes, escalations and increases from each business unit. It also includes a sales roster for each business unit.
If you need hypo contact info please reach out to Heather Burkett with part numbers and where you are located.
Communication Type:
Allocation Notification/Update
V#10125
Communication Type:
Sales Roster
V#10125
-
02/10/2025
Title: Greiner Bio-One V#11403
Details:
Discontinuation Notice

Communication Type:
Discontinuation Notice
V#11403
-
02/7/2025
Title: Hollister V#10901
Details:
Hollister Restore Hydrocolloids Product Discontinuation
Dear Valued Customer:
At Hollister, our mission is to make life more rewarding and dignified for those who use our products and services. In the spirit of achieving this mission, we may need to modify or discontinue some of our products.
Please be advised that the following Restore Hydrocolloids will be discontinued effective December, 31st, 2024, or until inventory is depleted.
Communication Type:
Discontinuation Notice
V#10901
-
02/7/2025
Title: Nestle V#10183
Details:
Usage Peptamen Jr Vivinex Complete
the products in the 1000 ml we will be keeping the 250 ml version plus providing some additional products in closed systems to pick from. The tentative out of stock date will be May-July of 2025. Listed below are the customers that you service and what products they are purchasing. I sorted according to volume
Rush SYS-Compleat Standard 1.4 1000 ml -318 case
UNIV OF KY HOSPL-Peptamen Jr 1000 ml 134 cases, Peptamen Junior 1.5 1000 ml 75 cases, Compleat Standard 1.4 1000 ml 60 cases
LOMA LINDA UNIV MED CTR (LLUHC)-Vivinex RTF 1000 ml 57 cases
UPSTATE UNIV HLTH SYS (SUNY)-Vivonex RTF 1000 ml 43 cases, Peptamen Jr 1.5 1000 ml 11 cases, Glutasolve 3 cases
ST LUKES-Vivonex RTF 1000 ml 33 cases
CHCA: EAST TENNESSEE (ETCH)-MCT Oil 14 cases and Peptamen Junior PHGG 16 cases
APPALACHIAN RGNL HLTHCARE (ARH)-Vivonex RTF 1000 ml 21 cases
WOOD CNTY HOSPL-Vivonex RTF 1000 ml 7 cases
ST LUKE S NAMPA MEDICAL CTR-Vivonex RTF 1000 ml 5 cases
MID MI MED CTR-Vivonex 1000 ml 2 cases
Home of The Innocents-MCT Oil 1 case
ROGERS MEM HOSPL-MCT Oil 1 case
If you have any questions please let me know
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Experience our Support Site
Communication Type:
Discontinuation Notice
V#10183
Communication Type:
Product Update
V#10183
-
02/6/2025
Title: Kate Farms V#12409
Details:
We are excited to announce the expansion of the Kate Farms® pediatric portfolio with four new products:
Pediatric Peptide 1.0 in Strawberry and Kids Nutrition in Vanilla, Chocolate, and Strawberry flavors.
At Kate Farms, our mission is to make nutrition the foundation of human well-being. Our innovative,
plant-based, organic products are designed to provide high-quality nutrition and are made without
common allergens or artificial sweeteners, flavors, colors, or preservatives — making them suitable for a
wide range of patients.
Our pediatric portfolio is designed to help support children’s growth and development and is eligible for
insurance coverage. The new Pediatric Peptide 1.0 in Strawberry is one of our most requested products,
offering another great-tasting peptide formula for tube feeding and oral use. Kids Nutrition is available in
three delicious flavors for oral nutrition supplementers, may also be used for tube feeding, and contains
DHA from a plant-based source.
AvailableCommunication Type:
New Product Launch
V#12409
-
02/6/2025
Title: Nestel V#10183
Details:
Attached is the Nestle inventory letter for this week.
- Boost VHC Strawberry was added to the report
- Boost VHC Chocolate was removed
- Impact Peptide 1.5 1000 ml was added to the report
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Experience our Support Site
Communication Type:
Product Inventory Update
V#10183
-
02/6/2025
Title: NDC V#10030
Details:
NDC/Exprecia Coagulation flyer
I wanted to announce a new product line that we have brought in through the NDC warehouse. It is the Xprecia Prime – Portable Coagulation monitoring device. This is a new product to the USA and NDC is the first master distributor to partner with Universal Biosensors to offer this to this to distribution. One of the features that I thought was impressive was that there is no separate chip required like the competition and everything is integrated into the strip with the barcode scanner built into the unit. They are offering a starter pack right now where you get 100 test strips along with the analyzer for only $500. Normally this would be $675 if your purchased the analyzer and test strips separate. All of the pricing is at the bottom of this email.
Please let me know if you would like me to connect you with the representative from Universal Biosensors so they can explain to you how this would be a good product to add to your offerings to compete with Roche and Coagusense.
Xprecia Prime™ – Portable Coagulation Monitoring Device
New Warehouse Program
Xprecia Prime™ is the new and improved generation of portable coagulation monitoring device. Xprecia Prime™ is small, accurate, and easy to use. This new coagulation analyzer is designed for fast and reliable PT/INR testing, at the point of care (POC), primary care, urgent care, and hospitals. Xprecia Prime™ meets the modern high-quality POC coagulation testing requirements, providing PT/INR results comparable to laboratory analysis in under a minute.
Next Generation cost effective analyzer with no long-term contracting requirements. Integrated bar code scanner with strip eject button for safe and easy disposal. No calibration chips and less blood required than existing analyzers.Xprecia Prime Analyzer
Xprecia Test Strips (4 x Vial of 25)
Xprecia Prime LQC
Xprecia Prime Starter Pack (1x 90306, 1x30106,1x90506)
NDC Program Details:
NDC Warehouse Program
Available NDC Medical & MedPlus
Available in the US ONLY
CPT Code/Description: CPT 085610
National Average Reimbursement: $4.29
Markets: Physician/Clinic, Acute Care/Surgery Center, Long Term Care/Post-Acute, Hospital, Nursing Home, Laboratory, Home Health Care, Government, Emergency – EMS, Veterinary
Contact: Tiffany Martin, US Commercial Director (916) 626-1151
Additional Resources:
Visit Xprecia Prime™ Product Page
Download Xprecia Prime™ Sell Sheet
Download UBI Sales RosterScott Berndt | National Accounts Manager
402 BNA Drive, Suite 500 | Nashville, TN 37217
direct: 615-366-5984
cell: 920-420-5187
website | facebook | twitter | linkedin
Yellow Belt Certified
Communication Type:
Product Flyer
V#10030
Communication Type:
New Product Launch
V#10030
-
02/5/2025
Title: Medtronic V#10255
Details:
Customer Listing_Medtronic allocation update
Concordance Team,
Medtronic announced allocation changes on the following items. Our system now reflects these changes.
- E7507 (CHS#699587) – increased the 70% allocation to 90%
- E7507-DB (CHS#164418) – increased the 70% allocation to 90%
- E7509 (CHS#101266) – removed the allocation
The customer listing is attached for reference.
Communication Type:
Allocation Notification/Update
V#10255
-
02/3/2025
Title: Hollister V#10901
Details:
Anchor Fast allocation letter and customer usage
1/24/2025
Dear Valued Business Partner,
On December 20, 2024, Hollister issued a voluntary recall in the United States and Canada on select lots of the AnchorFast™, AnchorFast Guard™, AnchorFast Guard Select™ and AnchorFast SlimFit™ Oral Endotracheal Tube Fastener due to reports of decreased skin barrier wear time.
After a thorough investigation, Hollister has taken corrective action and is resuming production of AnchorFast products with an updated product design to address the decreased skin barrier wear time.
While we are increasing production of AnchorFast products to minimize backorders caused by the recall, we also expect higher than normal order quantities.
To treat all our customers consistently, we are implementing thresholds on order quantities and placing our AnchorFast Oral Endotracheal Tube Fasteners 9799 on allocation for the foreseeable future.
This allocation will allow us to continue to support our customers with available inventory, while monitoring product demand.
Your weekly order threshold is: 75bxs
Please reach out to your Hollister representative if you have questions. Thank you again for being our valued business partner and for your understanding in this matter.
Sincerely,
Michelle
Communication Type:
Allocation Notification/Update
V#10901
-
02/3/2025
Title: PDI V#10420
Details:
Nice n Clean letter Customer usage
January 23, 2025
Nice ’n Clean® Scented Baby Wipes Discontinuation:
ATTN: Valued Distributor Partners and Customers,
After careful consideration, PDI has decided to discontinue our Scented 80 and 40 count Nice ’n Clean Baby Wipes (SKUs: M225XT, Q34540). For replacement options, please see our recommendations detailed below.
Discontinued:
Replace with auto-sub:
M225XT
M233XT
Nice ’n Clean Scented Baby Wipe 80ct
Nice ’n Clean Unscented Baby Wipe 80ct
Q34540
Q70040
Nice ’n Clean Scented Baby Wipe 40ct
Nice ’n Clean Unscented Baby Wipe 40ct
Replacement Option Specification Information:
PDI is grateful for your support and understanding. If you have any questions, please contact your Territory Sales Manager or our Customer Care Team at 1-800-999-6423.
Best Regards,
Briana Flanagan
Sr. Marketing Manager, Interventional & Patient Care Manufacturer Item # Product Name Wipe Size Packaging UOM Case Weight Case Cube Pallet TI/HI
M233XT
Nice ’n Clean Unscented Baby Wipe 80 Count
8.0” x 6.6”
80 wipes per pack/
12 packs per case
12.69 lbs
.752 ft
15/4
Q70040
Nice ’n Clean Unscented Baby Wipe 40 Count
8.0” x 6.6”
40 wipes per pack/
12 packs perCommunication Type:
Discontinuation Notice
V#10420
-
02/3/2025
Title: Siemens V#10030
Details:
Customer Facing Promo Lead Promo Distributor Pricing
Please see the attached 2025 promos—one customer-facing and one with the transfer price—along with the part item list. Nothing has changed.
Every situation is unique, and I’m happy to work with you and the customer on discounted pricing, aggressive LVCs, or other options depending on the opportunity to after competitive business. The big thing is that we don’t quire volume contracts and we offer the best prices.
Let me know if you have any questions!
Communication Type:
End User Promo
V#10030
-
02/3/2025
Title: Abbott V#10575
Details:
Dear Distributor Partner –
Abbott Nutrition is proud to offer your patients more variety when it comes to nutrition for wound healing. This spring, we’re releasing an exciting new flavor of Juven: Pineapple Coconut. This flavor offers the same nutrition for wound healing you’ve come to expect from Abbott, now with a tropical flavor that complements our existing Juven Orange, Fruit Punch and Unflavored portfolio. In addition to the launch of Pineapple Coconut, all Juven SKUs will have updated labels. There are no changes to the current Juven SKU numbers, UPC or NDC format codes. All items will retain the same packaging dimensions as existing products.
We estimate the availability of Pineapple Coconut to begin in late March 2025. New labels for existing flavors are expected to flow through in the following 1-2 months.
These items are being added to your Distributor/Wholesaler acquisition cost contract pricing as set forth in the table below:
Distributor/Wholesaler Acquisition Cost (3)
Item
Current Item #
NEW Item #
New Item UPC (Case)
New Item UPC (Unit)
New Item NDC Format Code (Unit) (1)
Estimated Launch Timing (2)
1 - 124 Case Price
125+ Case Price
Juven Pineapple Coconut 30ct
N/A
69032
659781690322
659781690308
59781-0690-32
Late March 2025
$71.50
$61.50
Juven Pineapple Coconut 6-30ct
N/A
69033
659781690339
659781690308
59781-0690-33
Late March 2025
$359.20
$349.20
(1)NDC-format codes: NDC Format codes are product codes adjusted according to standard industry practice to meet the format requirements of pharmacy and health insurance computer systems. Abbott Nutrition does not represent these products to be drugs, nor their codes to be NDC codes. If used for third-party billing purposes, each healthcare provider or contracted vendor is ultimately responsible for the accuracy and appropriateness of codes billed for individual patients and services.
(2) Estimated launch timing is subject to change.
(3) 125 Cs Price / Case: All Abbott products have bracketed pricing based on the quantities ordered which is the price that is reflected on distributor's invoices from Abbott. For Distributor/Wholesaler Acquisition Cost contracted pricing, the 125-case contract price is your Cost-Basis for chargeback claim submissions to Abbott (outlined in red).
The attached Customer letter is being distributed to our mutual end-user customers beginning this week with direction for them to work directly with their distributor representative on product availability for these items.
Please let us know if you have any questions.
Distributor Relations Contact Information:
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Nick Saffell; Sr. Business Analyst: email: nicholas.saffell@abbott.com; phone: 614‐624‐6446
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Joe
Communication Type:
New Product Launch
V#10575
-
02/3/2025
Title: Greiner V#11403
Details:
Dear Greiner Distribution Partners,
I hope this message finds you well. I am writing to inform you that Greiner Bio-One will be discontinuing item 455083P VACUETTE® TUBE 8 ml LH Lithium Heparin Separator, 16x100 green cap-yellow ring, non-ridged tube, effective May 1, 2025, or once stock is depleted, whichever occurs sooner.
Alternatively, we offer the 456087P VACUETTE® TUBE 5ml LH Lithium Heparin Separator, 13x100 green cap-yellow ring, non-ridged. I kindly request that you remove item 455083P from your listings effective May 1, 2025.
You will find the customer notification we are sending to customers and distributors attached for your reference.
Thank you for your attention to this matter.
Terry
________________________________________________________
Terry Prymek
Key Account ManagerGreiner Bio-One North America, Inc.
4238 Capital Drive · Monroe · NC · 28110
M: 704-254-6211
Terry.Prymek@gbo.com · https://url.avanan.click/v2/r01/___www.gbo.com___.YXAzOmNvbmNvcmRhbmNlaHM6YTpvOjczMTYwNzI2YThmYWM2N2Q1ZGJjNGZjMjVjYjdmZGRlOjc6ODcyNjo4NDUyMzQ5MjQ4ZGMwYTE5YjMyMGVhMjIyNmQ5MGZkNjlmMjA4NWU4MWVhNjY3YzQ3ZGRiMDkzZDMwNDEyY2E1OnQ6RjpG
Communication Type:
Discontinuation Notice
V#11403
-
01/29/2025
Title: Stryker Ins V#10912
Details:
Vendor Item List Sales History 7.28.24-1.28.25
Concordance Team,
Stryker V#10912 announced that mfr #0406-650-205 will be discontinued. The usage file is attached. If we have an active item equivalent, the team will reference it for#1089757 in VAI.
Communication Type:
Discontinuation Notice
V#10912
-
01/28/2025
Title: Nestle V#10183
Details:
Thicken Up Discontinued items Usage file
Good Morning.,
I’m following up on the below communication, it announced the discontinuation of the Original Thickenup powder. The announcement was sent at the end of October in 2023, the depletion date for the inventory was the end of December. This did take a bit longer and the ETA is now Mid-February. Should you have any questions please let me know
Communication Type:
Discontinuation Notice
V#10183
-
Communication Type:
Supply Disruption
V#10801
-
01/28/2025
Title: BD V#10125
Details:
S#311528 Mfg#10015414 has been discontinued and suggested sub S#180832 Mfg#10062818. S#311528 has been placed in item inactivation.
Please see attached notification from BD.
Please see customer listing and usage for the past 3 months in the attachment.
If you have any questions, please reach out to Heather Burkett.
Communication Type:
Discontinuation Notice
V#10125
-
01/28/2025
Title: HOM V#10075
Details:
Vendor Item List Sales History 7.28.24-1.28.25
Concordance Team,
Health-O-Meter requests that this highlight be given to our Sales Team:
Why NTEP Certification is Unnecessary for Medical Scales
When it comes to medical equipment, ensuring compliance with the right certifications is crucial for safety and performance. While some may advocate for NTEP certification, it’s important to recognize that this certification is primarily for commercial weighing devices and doesn’t address the specific needs of medical settings.
Key Certifications for Medical Equipment:
- ISO:13485: A globally recognized standard for quality management systems in the medical device industry.
- MET Certification: Signifies that equipment meets international standards for safety and performance.
Medical scales adhere to ISO:13485 and MET certifications, ensuring accuracy, safety, durability, and patient risk management. These standards include the same relevant accuracy testing as NTEP, with added focus on medical use. Adding NTEP certification can increase costs and complicate equipment with unnecessary features and workflow changes, without providing additional clinical value. No standard can ensure accuracy in the field. Regular maintenance and calibration are essential for all scales, regardless of the standards they meet.
At Health o meter® Professional Scales, we prioritize ISO:13485 and MET certifications to ensure our products deliver the highest quality and performance in healthcare settings.
Click here to learn more about why NTEP certification may not be necessary for medical scales. For any questions or more information, reach out to Brett Lucido, Director of National Accounts, blucido@homscales.com, (724) 799-4233.
Communication Type:
Updated Supplier Information
V#10075
-
01/27/2025
Title: Baxter V#10797
Details:
Customer Listing_from Casey_1.27.25
Concordance Team,
Baxter issued the attached allocation letter in regards to IV products.
- Keep in mind that non-VA customers do not use the imported products that are listed as substitutions in the letter’s matrix.
- Baxter increased allocations to 100% for #2B7127 (C #605261) and #2B1064X (C #781211) but to achieve the 100% involves a combination of these Baxter codes + corresponding import codes (JB7127 + A6C1064US, respectively) found in the matrix.
- The customer listing for #2B7127 (C #605261) and #2B1064X (C #781211) is attached.
Message from Casey after she updated allocations in the system: I did not move allocations over to the subs automatically for any customer where the allocation is split into groups. Some customers have already approved and added in the subs and have allocation. If any customer decides they would like to use the other item, please have the AM/CS let me know and we can get them added in.
Communication Type:
Allocation Notification/Update
V#10797
-
01/27/2025
Title: Baxter V#10797
Details:
Vendor Item List Sales History 7.26.24-1.26.25
Concordance Team,
Baxter is discontinuing item #2B7477 (C #156969).
---There are substitutions listed in VAI.
---Open POs for this item still remain in the system until Baxter sends a notification that they have depleted their inventory. Then our expeditor will close any orders that will not be filled.
Communication Type:
Discontinuation Notice
V#10797
-
01/27/2025
Title: NeotechV#10387
Details:
Neotech has not been negatively affected by recent California wildfires. We remain open with all products fully stocked and ready to ship.
Email not displaying correctly?
View it in your browser.Shipping Daily Monday-Friday!
Dear Mary,
We're sending this note to inform you that Neotech Products is fully operational. The SoCal wildfires have not hindered us in any way.
All products are in stock and ready to ship!
Or Call 1-800-966-0500 to Speak with a RepresentativeProduct Catalog
Neotech Products has emerged as an essential brand in hospitals and homes around the world. Learn more about all of the products we offer.
Clinical Training
Neotech employs an experienced team of licensed Clinical Consultants who work with hospitals providing educational support and in-service training. Clinical Consultants can provide onsite in-service training for your staff including night and weekend shifts.
Call 1-800-966-0500 or Connect with Us
Copyright © 2025 Neotech Products LLC. All rights reserved.
Our mailing address is:
Neotech Products
28430 Witherspoon Parkway
Valencia, CA 91355
U.S.A.Communication Type:
Updated Supplier Information
V#10387
Communication Type:
Weather Impact Update
V#10387
-
01/27/2025
Title: Nestle V#10183
Details:
Attached is the Nestle inventory letter for this week. The Adult and pediatric versions of Compleat Organic Blends are still on the list
- Boost VHC Chocolate was added to the letter
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.comCommunication Type:
Product Inventory Update
V#10183
-
01/24/2025
Title: Abbott V#10575
Details:
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Joe
Joe Corey
Sr. Business Analyst
Abbott Nutrition
Commercial Operations
Abbott
3300 Stelzer Rd.
RP 2-3
Columbus, OH 43219
O:
+1 614-624-6600
F:
+1 614-727-3896
Communication Type:
Product Inventory Update
V#10575
-
01/23/2025
Title: Amsino V#10385
Details:
Updated Sales Roster

Communication Type:
Sales Roster
V#10385
-
01/23/2025
Title: Medline V#10605
Details:
Extended lead times for our midstream kit (DYND30212)
We are currently facing extended lead times for our midstream kit (DYND30212) due to a BZK towelette shortage.
We expect to improve our stock levels by the end of July and will keep you updated on any changes to that timeline.
Please see below for suggested substitute and the differences between sub and original:
DYND30224 KIT,MIDSTREAM,HANDLE/BZK,4OZ CUP,VALUE
Please note that this item features a collar rather than a full funnel as well as a standard cup that is not 95kPa certified.
Sub is also 100 EA/CS whereas original item is 40 EA/CS.
See below for overview of suggested sub

Communication Type:
Backorder Notification/Update/Reports
V#10605
-
01/22/2025
Title: BD V#10125
Details:
BD Hypodermic Allocation Adjustments January 2025
Please see attached from BD regarding allocation adjustments and updates. Please let me know if you have any questions. Thanks,

Communication Type:
Allocation Notification/Update
V#10125
-
01/21/2025
Title: BD V#10125
Details:
Discontinuation notice for C#239373 is attached here.
There are no stock customers using this so usage and customer listing was not ran. Mfg.# 367364 (suggested alternative) is on allocation, however, there is stock available. Please reach out to Heather Burkett if you have any questions. Thanks,
Communication Type:
Discontinuation Notice
V#10125
-
01/21/2025
Title: Coloplast V#10581
Details:
Vendor Item List Sales History 6.21.24-1.21.25
Concordance Team,
Reminder from Coloplast V#10581: “The US Government has imposed economic and trade sanctions on Russia and Belarus in response to Russia’s 2022 invasion of Ukraine. In support of these sanctions, this is a reminder to our distributors to maintain strict compliance with your existing contractual obligations to not resell any Products for distribution, sale, or use outside of the United States or its territories and possessions.”
Communication Type:
Updated Supplier Information
V#10581
-
01/20/2025
Title: S2S V#10006
Details:
Dear Valued Distributor Partners, FAQ and Strategic Partnership
As part of the strategic partnership between S2S Global and DeRoyal, the Sales Team has become part of the DeRoyal sales network. As a result, you will have additional support from the expansive DeRoyal team in addition to your current S2S team to provide you exceptional customer support. There are no changes to the S2S products, categories, and/or contracts moving forward. Our team will continue to support you and the S2S portfolio as we did before.
As we continue to move forward with our strategic partnership, we wanted to provide you with some additional information and to answer some frequently asked questions. We look forward to continuing our valued relationship and to providing additional support to you and your needs.
Communication Type:
Updated Supplier Information
V#10006
-
01/20/2025
Title: Nestle V#10183
Details:
Nestle inventory letter for next week.
- Boost Plua vanilla was removed
- Boost VHC Chocolate was removed
- Diabetisource AC was removed
- Compleat Organic Blends Plant Based was added
- Compleat Organic Blends Pediatric was added
- Compleat Pediatric Peptide 1.0 was added
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Experience our Support Site
Communication Type:
Product Inventory Update
V#10183
-
01/20/2025
Title: Vantive V#13578
Details:
Vendor Item List Sales History 6.20.25-1.20.25
Concordance Team,
Item #266874 (mfr # 106957S) has been removed from allocation in the system after receiving notification from Vantive V#13578 that it has been lifted.
The usage report is attached for reference.
Communication Type:
Allocation Notification/Update
V#13578
-
01/20/2025
Title: Gerson Medical V#10725
Details:
Vendor Item List Sales History 6.20.24-1.20.24
Concordance Team,
Gerson Medical V#10725 announced in the below table that for standard production of item #065000 (C #349904), the flash drive is being replaced with a QR code. The item number and purchase price remain the same. The vendor stated, “For customers who wish to continue purchasing the fit test kits (065000 or 066000) with the flash drive, additional costs will be incurred. Please contact custserv@gersonco.com for further information.”
The usage file is attached.

Communication Type:
Product Changes
V#10725
-
01/19/2025
Title: Stryker V#10912
Details:
Concordance Team,
Item 5450-800-308 (C #236375) is discontinued per Stryker Instruments V#10912. They have no recommended subs at this time, but a 12-month usage report was ran and there were no Concordance sales during that time period.
Communication Type:
Discontinuation Notice
V#10912
-
01/17/2025
Title: Abbott V#10575
Details:
Abbott Product availability
We are sharing with all of our distributor partners the attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Joe
Joe Corey
Sr. Business Analyst
Abbott Nutrition
Commercial Operations
Abbott
3300 Stelzer Rd.
RP 2-3
Columbus, OH 43219
O:
+1 614-624-6600
F:
+1 614-727-3896
Communication Type:
Product Inventory Update
V#10575
-
01/16/2025
Title: Ecolab/ Mircotek Medical Inc. V#10057
Details:
Due to the extended recovery date of the affected C9 Drape packs, I wanted to give you an alternative until we can recover supply which is currently anticipated Mid 2025. I have included alternatives to the contents in the packs as well as the case packs. Please let me Heather Burkett know if you have any questions.
C9-006M
CONTENTS
CASE QTY
20/CS
ALTERNATIVE SKU
CASE QTY
MINI C-ARM DRAPE FOR OEC 6600
C9-106M
20
30/X24 KEYBOARD COVER
KB1277
40
14X30 FOOTSWITCH DRAPE
2420FSN
50
C9-008M
CONTENTS
CASE QTY 20/CS
ALTERNATIVE SKU
CASE QTY
MINI C-ARM DRAPE FOR OEC 6800
C9-108M
20
30/X24 KEYBOARD COVER
KB1277
40
14X30 FOOTSWITCH DRAPE
2420FSN
50
Communication Type:
Backorder Notification/Update/Reports
V#10057
Communication Type:
Substitution Option
V#10057
-
01/16/2025
Title: Medline V#10605
Details:
This notice is to inform you of a current backorder situation regarding the FitRight large size extended wear protective underwear (MSC53505)
Timeline
We are expecting to have healthy inventory of MSC53505 in March 2025. In the meantime, please sub with the sku below:
SUB: MSC33505 UNDERWEAR, PROTECTIVE,SUPER,L,4/18S

Communication Type:
Substitution Option
V#10605
Communication Type:
Supply Disruption
V#10605
-
01/15/2025
Title: Welch Allyn V#10834
Details:
BP Cuff Supply Update_Customer Communication_Jan7_2025
Concordance Team,
Welch Allyn announced that several BP cuff product categories have come off allocation as they are very healthy in those specific cuff types (5-10 day lead time). From our inventory analyst team about the updated allocations in our system:
* Items notated on the customer communication letter were removed from allocation.
* BP cuff spreadsheet attached of those CHS #s through WA and VHSS (2 separate files) and customer listings as well.
The supplier stated they are on course to reach their goal of getting back to normal lead times at the end of Q1.
Please use the attached letter as a reference if customer questions arise.
Communication Type:
Allocation Notification/Update
V#10834
-
01/15/2025
Title: TSC Life V#13603
Details:
Vendor Item List Sales History_Usage 1.10.24-1.10.25_Mistral Air
TSC Life-Mistal Air item numbers currently set up in VAI with CHS item numbers 1.10.25
Concordance Team,
Mistral Air items are manufactured by TSC Life (v#13603).
- TSC Life changed their numbering system, so we are advising that you let your customers know to update their systems accordingly.
- Manufacture item numbers used to have a -PM at the end. TSC Life has removed the -PM.
- Concordance item numbers remain the same.
- For the Mistral Air items currently set up in our system, the IM team has updated the revised manufacturer item numbers (VAI and web are updated).
- A usage report is attached for your reference.
For example, C #274383 crosses to MA3360 which used to be MA3360-PM:

Communication Type:
Product SKU List
V#13603
-
01/14/2025
Title: Essity V#10272
Details:
Essity Product Announcement regarding NEW Tena Dry Comfort Pads
Please see attached product launch
Please see attached Pricing info
Please see attached operations info
If you have any questions, please reach out to Heather Burkett. Thanks,
Communication Type:
New Product Launch
V#10272
-
01/14/2025
Title: Medline V#10605
Details:
DYNJAAMASK3 has been discontinued. This cancels all active DEDs for this item.
Below are the sub options.
DYNJAAMASK33 MASK,FLEXIBLE,ANESTH,SIZE 3,TOP VALVE
DYNJAAMASK43 MASK,ANESTH,SIZE 3,TOP VALVE
Please reach out to Heather Burkett if you have any questions. Thanks,
Communication Type:
Discontinuation Notice
V#10605
-
01/12/2025
Title: Medline V#10605
Details:
Medline MBD200 Baby Diaper Conversion
Please see attached file from Medline and Customer Listing with usage. This has also been sent via email to Item Maintenance, Buyers, Account Managers and Customer Relations team.
This notice is to inform you that the current Medline branded baby diaper series (MBD200) will be going through the Item Conversion process.
Reason
The reason for this conversion is due to an upgrade for our Medline branded baby diapers.
The new Medline branded baby diaper series (MBD300) will have updated packaging artwork, an updated diaper design, and an upgrade in performance. Please see the attached documents regarding the change:
Timeline for Change
Please review the following table of important dates for this change:Milestone Key Date
MBD200N Run Out Date
September 2025
MBD2001 Run Out Date
Mid-February 2025
MBD2002 Run Out Date
Mid-February 2025
MBD2003 Run Out Date
March 2025
MBD2004 Run Out Date
April 2025
MBD2005 Run Out Date
March 2025
MBD2006 Run Out Date
March 2025
MBD2007 Run Out Date
March 2025
Action
Forecast has already been shifted to the new item. Your customer can continue to purchase the current item until stock is depleted.
Please contact Heather Burkett if you have any questions. Thanks,
Communication Type:
Discontinuation Notice
V#10605
-
01/12/2025
Title: Medline V#10605
Details:
Hypodermic needles- Prolonged Distribution
Please see below, attached from official notice Medline and Concordance customer listing with usage. This has also been sent via email to account managers and customer relations. If you have any questions please reach out to Heather Burkett. Thanks,
Due to unforeseen manufacturing constraints in addition to heightened demand during flu season we are expecting prolonged distribution on several hypodermic needles.
As a result, we will be placing the below standard needle SKUs on limited allocation. Allocation was given to customers based on historical usage.
- Sub Options: There are subs available, which are listed in the attached file.
- Outlook: We are expecting full recovery mid-April (although some skus are expected to recover sooner) “get well” dates included in the table below. We are working to expedite our highest volume skus and will continue to reevaluate allocation and will increase as able.
Items on Allocation as of 1/7/2025
Communication Type:
Allocation Notification/Update
V#10605
-
01/10/2025
Title: Nestle V#10183
Details:
Attached is the Nestle Inventory Letter for next week.
- Boost VHC Vanilla was removed
- Boost VHC Strawberry was removed
- Boost VHC Chocolate was added
- Compleat Standard 1.4 was removed
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Experience our Support Site
Communication Type:
Product Inventory Update
V#10183
-
01/9/2025
Title: Welch Allyn V#10834
Details:
Exam Tools Trade Up Promotion Flyer-LR (1)
RetinaVue Care Delivery Model Promotional Flyer-LR
Spot Vision Screener Promotional Flyer-LR
Vendor Item List Sales History 7.8.24-1.8.25
Concordance Team,
Take a look these Welch Allyn National Promotions for Q1. Please share the attached flyers with customers and refer to the fine print for important details.
A 6-month usage report is attached along with a summary matrix that lists both the manufacturer and Concordance item numbers.
- Spot Vision Screener - $1,500 rebate with the purchase of the device & 1-year SmartCare Service Plan
- RetinaVue - $500 rebate with the purchase of the device
- Exam Tools Trade up Offer- End-user rebate when "trading up" legacy Welch Allyn devices to our newer “BEST” configurations
--Rebates on “BEST” configurations only
--Customers DO NOT need to send in old devices but need to provide a picture of what "they're trading in" on the rebate form
--Please note- 2 new IWS model numbers have been added
- Spot Vision Screener Trade-Up - $1,000 Spot Vision Screener Trade-Up until the end of June
Communication Type:
End User Promo
V#10834
-
01/9/2025
Title: ICU Medical V#10642
Details:
MX850 Tamper Resistance Set Discontinued
Dear Valued ICU Medical Customer:
We want to thank you and all our customers for the overwhelmingly positive response to the value and promise our
combined portfolio of clinically relevant products brings you and your patients.
This letter aims to inform you of the discontinuation of item MX580, tamper resistant extension set, effective
immediately. All unfulfilled purchase orders with ICU Medical for the discontinued item will be cancelled.
While we had to make some complex choices in this regard, we apologize for the short notice and the potential
impact this may have on you. We do not undertake such actions lightly as we consider the patient first and foremost
in all decisions. Please work with your sales representative on a possible substitute.
This notice does not affect orders for any other products or services except as detailed above.
We sincerely appreciate your business and will keep you informed of any further integration changes. Please
contact your sales representative or Customer Service at customerservice@icumed.com with any questions
regarding this letter.Communication Type:
Discontinuation Notice
V#10642
-
01/9/2025
Title: ICU Medical V#10642
Details:
Important - Small Volume Quad-for-Single Pack Substitutions for Sodium Chloride and Dextrose IV Solution
Concordance Team,
Attached is a customer notification letter regarding a supply constraint affecting our small-volume IV solution bags, including the following:
- 50mL Normal Saline
- 100mL Normal Saline
- 50mL Dextrose 5%
- 100mL Dextrose 5%
This constraint primarily impacts individually packaged bags. As a substitute, we may provide the Quad-pack variation with an equivalent quantity of units based on your allocation.
If you have any questions please reach out to Heather Burkett. Thanks,
Communication Type:
Allocation Notification/Update
V#10642
Communication Type:
Backorder Notification/Update/Reports
V#10642
Communication Type:
Substitution Option
V#10642
Communication Type:
Supply Disruption
V#10642
-
01/9/2025
Title: Nestle V#10183
Details:
Attached is the first inventory report for 2025.
- Boost VHC Vanilla was added
- Boost VHC Strawberry was added
- Diabetisource AC 1500ml was added
- Compleat Pediatric Standard 1.4 was removed
- Boost VHC Chocolate was removed
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Experience our Support Site
Communication Type:
Product Inventory Update
V#10183
-
01/9/2025
Title: AbbottV#10575
Details:
We are sharing with all of our distributor partners the attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Joe
Joe Corey
Sr. Business Analyst
Abbott Nutrition
Commercial Operations
Abbott
3300 Stelzer Rd.
RP 2-3
Columbus, OH 43219
O:
+1 614-624-6600
F:
+1 614-727-3896
Communication Type:
Product Inventory Update
V#10575
-
01/8/2025
Title: Molnlycke V#10008
Details:
Distributor Letter US T&P 1401008 Discontinuation 12-18-2024
Z Flo 1401008 Upgrade Chart 12-18-2024
Concordance Team,
The supplier announced that manufacturer item #1401008 (C #239698 & govt C #399192) is being discontinued effective March 31, 2025. They have direct replacements for the product, packaged without the cover, at a more attractive price point. Moving forward they are providing customers with the ability to order Z-Flo covers for this SKU separately in both washable and disposable forms. Please review to the attachments for more information.
A 6-month Concordance usage report for the item was ran which revealed zero sales during that time frame.
Communication Type:
Product Flyer
V#10008
Communication Type:
Discontinuation Notice
V#10008
-
01/7/2025
Title: Stryker V#10912
Details:
ENT Products (Nasopore and Hemopore) REMINDER
Concordance Team,
Stryker Instruments V#10912 sent a reminder that ENT products (Nasopore and Hemopore) always require pre-approval from local Stryker Sales Reps. These products require pre-approval for each individual end user before dropship orders can be placed. Customers will need to work with their local Stryker Sales Representatives to obtain pre-approval. These products cannot be shipped to Distributor warehouses or distribution centers due to this required pre-approval for all end-users.
Communication Type:
Updated Supplier Information
V#10912
-
01/7/2025
Title: Health-O-Meter V#10075
Details:
Vendor Item List Sales History 7.1.24 - 1.2.25
Concordance Team,
Health-O-Meter V #10075 sent an announcement about their Wheelchair Scale Accessibility and Compliance Guide.
- Having the right wheelchair scale is essential to ADA compliance and creating an inclusive space.
- The guide is a resource to help customers understand regulations, best practices and how to choose the right scale.
- Click on this link to access the guide: Download the Guide Now
- The guide references a retrofit kit for the existing 2600KL and 2610KL series of scales.
--However, the retrofit kit is not yet available. The supplier will send the product information and pricing once it is available. - The supplier advises that customers fill out the form on the provided webpage link to be contacted about availability of ADA-Compliant Wheelchair Scales.
Communication Type:
Updated Supplier Information
V#10075
-
01/6/2025
Title: 3M SIBG V#13559
Details:
Vendor Item List Sales History 7.6.24-1.6.25
Concordance Team,
C #326100 (3M catalog #310-1060) has been discontinued by the supplier. No substitution is currently listed in our system, but the supplier did list catalog # 310-1001 as a suggested alternate. If there is future customer demand for this alternate item, let the IM team know if the item needs set up in the system.
Communication Type:
Discontinuation Notice
V#13559
-
01/3/2025
Title: Central Solutions V#11992
Details:
discontinued
Dear Valued Customers, Customer usage
I am writing to inform you of upcoming changes to our product line up. Due to several factors, we have made the difficult decision to discontinue certain products and/or SKUs. We apologize for any inconvenience these shifts will make. However, to help with this transition we have alternatives listed below for your consideration. Please note that we will continue to carry the discontinued items as we sell through stock, however they will be consolidated once stock is fully depleted.
Part #
Description
Alternatives
14012
DC SANITIZER W ALOE 24X8OZ CAP
14013, 14017 (8oz, 18oz), DC Hand Sanitizer
23083
DC SKIN CARE CREAM
BOVI21004, BoaVida Recovery Crème
23182
DC VANILLA BEAN CREAM
BOVI21004. BoaVida Recovery Crème
23116-1250
DC ANTI-BACTERIAL HAND SOAP
14026-1250, DC Antimicrobial-P Hand Soap
23096
DC SKIN PROTECTANT CREAM
BOVI21024, BoaVida Canopy
Additionally, as indicated below we must adjust pricing on one of the SKUs in our product offering, DermaCen Foaming Lotion Soap (1390-1000). This will be affective on April 1st, 2024. We do not pass along price increases lightly and do understand the importance of offering a competitive price. However, this adjustment is necessary in order for us to remain a viable supplier to our customers.
Part #
Description
Current Price/CS
New Price/CS
1390-1000ML
DC FOAMING LOTION HND SP 4/CS
$31.64
$33.86
If you have any questions or concerns regarding this price adjustment and consolidation effort, please feel free to reach out.
-Henry
Office: 913.621.6542
Find us Online
Communication Type:
Discontinuation Notice
V#11992
-
Communication Type:
Label Changes
V#10078
Communication Type:
Updated Supplier Information
V#10078
-
Communication Type:
Updated Supplier Information
Vendor #10221
-
12/31/2024
Title: GE #10126
Details:
DINACLICK-compatibility-guide_-1 Black and -5 Gray Connectors_JB02857XX
Vendor Item List Sales History 6.30.24 -12.30.24
2024-11-25 ISO 80369-5 Standard Frequently Asked Questions JB11177US
Concordance Team,
GE Healthcare announced they are transitioning their CRITIKON™ non-invasive blood pressure cuffs from the ISO 80369-1 DINACLICKTM connection system to the ISO 80369-5 DINACLICKTM connection system. Our supplier contact explained:
- The timeline for this transition has begun and they look to be fully transitioned by early to mid-2025.
- It is best for customers to start transitioning to the new connector and associated BP cuffs.
- Please review the attached FAQs, the compatibility guide and part number list. (these are the first three attachments)
The usage file is attached for your reference.
This is NOT a discontinuation at this time. GE will send notifications about discontinuations of each item as they happen, but they recommend that customers start transitioning to the new items.
Communication Type:
Product Update
V#10126
-
12/31/2024
Title: Ecolab #10929
Details:
Ecolab Total Body Shampoo Communication December 2024
Vendor Item List Sales History 6.30.24-12-30.24
Concordance Team,
From Ecolab:
- Due to a regulatory change in New York state, Ecolab’s legacy Total Body Shampoo offering shall no longer be distributed, sold, offered, or exposed to sale specifically in the state of New York after December 31, 2024.
- In an effort to reduce supply chain disruptions, Ecolab has reformulated our TBS offering to comply with the state of New York and the updated formula entered the market during the month of December.
- The new formulation has the same benefits of the legacy product with no impact to product performance or use instructions.
- Distributors failing to comply with the regulatory change could be held liable for civil penalties, as indicated in the regulation (referenced in attached letter).
- The new formulation will begin entering the market starting December 2024. This will ensure that distributors will be allowed to distribute the new improved Total Body Shampoo formula without supply disruption.
- Said lot numbers permitted for sale in NY starting January 1, 2025 are in the attached communication.
- If a risk of sale is identified, disposition of any Total Body Shampoo products on hand that do not meet the following Lot Number criteria in the table below is recommended.
A 6-month usage file is attached.
-
12/30/2024
Title: TIDI V#10260
Details:
Re.: Product Discontinuation Notice – TIDI Emergency Blanket 980077
Please see link here for Discontinuation notice for C#411660/ 980077
This item is not stocked in any of our locations.
If you have any questions please reach out to Heather Burkett. Thanks,
Communication Type:
Discontinuation Notice
V#10260
-
12/23/2024
Title: Nestle V#10183
Details:
Weekly product update
Attached is the Nestle Inventory Letter for this week.
- Boost Kids Essentials Chocolate was removed
- Boost Kids Essentials Vanilla was added
- Novasource Renal 1000 ml was removed
- Nutren Jr Fiber was removed
- Compleat Pediatric Standard 1.4 was added
- Boost VHC Chocolate was added
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Experience our Support Site
Communication Type:
Product Inventory Update
V#10183
-
12/23/2024
Title: Abbott V#10575
Details:
We are sharing with all of our distributor partners the attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
Please note, the next Inventory letter will be sent on January 9, 2025.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Joe
Joe Corey
Sr. Business Analyst
Abbott Nutrition
Commercial Operations
Abbott
3300 Stelzer Rd.
RP 2-3
Columbus, OH 43219
O:
+1 614-624-6600
F:
+1 614-727-3896
Communication Type:
Product Inventory Update
V#10575
-
Communication Type:
Product Catalog
V#10901
-
12/23/2024
Title: Stryker V#10613
Details:
Stryker Integrity Matters newsflash - issue 4 2024
Concordance Team,
Stryker shared the attached announcement regarding integrity and ethics with regard to gifts and entertainment when interacting with healthcare providers.
Happy Holidays to all.
Communication Type:
Updated Supplier Information
V#10613
-
12/22/2024
Title: Stryker V#10912
Details:
Concordance Team,
Stryker Instruments announced the following discontinued items:
3000-002-000 --This SKU is being replaced by new SKU BLU62-8oz as of 11/27/24. The new SKU is a functional replacement, meaning that it looks like and produces the same results for the customer.
5400-701-000 -suggested substitute 5420-MED-000
5400-702-000 -suggested substitute 5420-FNE-000
5400-703-000 -suggested substitute 5420-PRP-000
5921-030-135NSc-no recommended subs available
5450-850-003 -alternative item 5500-573-000 is only compatible with the next generation Sonopet iQ system tubing set
5450-800-308 -no recommended subs available
2296-301-000 -no recommended subs available
Thank you.
Communication Type:
Discontinuation Notice
V#10912
-
12/22/2024
Title: Stryker V#10912
Details:
Vendor Item List Sales History with Units_Usage 6.17.24-12.17.24
Concordance Team,
Some items from Stryker Instruments V #10912 have been marked temporarily unavailable due to the reasons described below by the supplier:
- CRITICAL: 0210-008-000 FEM CANAL TIP IRRI/SUCTN: All open orders for this will be delayed at least 6 months due to limited manufacturing. Recommended sub is 0210-007-000.
- 0206-710-000 BIO PREP KIT and 0206-714-000 FEMORAL SPONGE WITH SUCTION: Currently delayed due to a minor supply disruption. Current ETA for non-dropship orders is Q1.
- 4118-135-105S1 DUAL CUT SAGITTAL BLADE: This product has delayed availability due to limited stock. Current ETA for non-dropship orders is Q1.
- 0606-563-000 REVOLUTION CMS W/BREAKAWAY: This product is facing delays due to its limited shelf-life not meeting the requirements to be shipped to distribution centers. If product is urgently needed, we recommend your end user reaches out to their local Stryker Sales Representative to place a direct order, since end users often have shorter shelf-life requirements and we do have plenty of stock available with shorter dating.
A usage report is attached for your reference.
Thank you.
Communication Type:
Temp Unavailable
V#10912
-
12/18/2024
Title: Abbott V#10575
Details:
Attached you will find the standard weekly inventory update letter you have been receiving:
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Joe
Joe Corey
Sr. Business Analyst
Abbott Nutrition
Commercial Operations
Abbott
3300 Stelzer Rd.
RP 2-3
Columbus, OH 43219
O:
+1 614-624-6600
F:
+1 614-727-3896
Communication Type:
Product Update
V#10575
Communication Type:
Allocation Notification/Update
V#10575
-
12/18/2024
Title: Phase Scientific V#12840
Details:
Spec Sheet- COVID OTC Tests
INDICAID® OTC is a non-invasive, rapid at-home test
that is an affordable, accurate, and convenient method of detecting COVID-19. INDICAID
OTC’s portability, ease-of-use, and capability for self-collection testing makes it one of the
most efficient ways to gain peace of mind for consumers and patients alike.Please click here to see spec sheet from Phase Scientific. Competitive pricing available.
Communication Type:
New Product Launch
V#12840
-
12/18/2024
Title: ICU Medical V#10642
Details:
EzPAP™ Airway Therapy Systems with Masks Product Discontinuation Notification
Please click here for official supplier letter PDF.

Communication Type:
Discontinuation Notice
V#10642
-
12/18/2024
Title: Bracco V#10034
Details:
New Varibar Mini Product Launch Setup Forms
New packaging solution enhances sustainability, workflow efficiency, and patient throughput
Princeton, NJ – Bracco Diagnostics Inc. (BDI), a U.S. subsidiary of Bracco Imaging S.p.A., one of the world’s leading companies in the diagnostic imaging business, and a pioneer in healthcare innovation, proudly unveils its latest breakthrough in barium solutions: the "Mini" Packaging for VARIBAR® (barium sulfate) oral suspension. Designed to meet the diverse needs of healthcare providers, this packaging option offers smaller volumes of VARIBAR products, the gold standard in Modified Barium Swallow Studies (MBSS).
Mini packaging will be available for VARIBAR® NECTAR (barium sulfate) oral suspension, VARIBAR® THIN HONEY (barium sulfate) oral suspension, VARIBAR® HONEY (barium sulfate) oral suspension, and VARIBAR® PUDDING (barium sulfate) oral paste.
Time-tested, trusted, and FDA-approved, the legacy of standardized viscosities of the VARIBAR product line helps eliminate the unpredictability of varied barium preparations.1-4 The "Mini" Packaging is a significant milestone as it enhances procedural efficiency and patient care.
"The VARIBAR line of products, with standardized viscosities, consistently addresses the most important needs in Modified Barium Swallow Studies," said Cosimo De Pinto, Senior Vice President of Sales and Marketing at BDI. "Now, with this new additional packaging solution, VARIBAR products are available to clinicians in sustainable, smaller volume options."
The key features of this innovative packaging include:
- Tailored Solutions: Healthcare providers can now access VARIBAR products in smaller quantities, optimizing resource utilization and reducing waste.
- Sustainability: BDI prioritizes using recyclable and biodegradable materials in manufacturing the "Mini" packaging, as it promotes a circular economy thereby reducing reliance on single-use plastics.
- Streamlined Workflow: The "Mini" Packaging facilitates smoother administration of VARIBAR contrast, enhancing workflow efficiency and patient throughput.
"This new packaging minimizes product waste, thereby reducing the environmental footprint of healthcare procedures," said Amy King, Director of Sustainability at BDI. "Compact packaging optimizes storage space and transportation efficiency, further reducing consumption and emissions."
By offering VARIBAR products in a more versatile and accessible format, BDI continues to invest in the future of abdominal radiology. The "Mini" Packaging for VARIBAR products is now available for order from all BDI wholesalers and distributors.
Please click links below for product information.
Communication Type:
New Product Launch
V#10034
-
12/18/2024
Title: Audit Microcontrols V#12466
Details:
Effective 12/18/2024, the product K956M-5 Linearity FLQ Special Diabetes for Abbott Systems has been discontinued.
There is a functional replacement, K889M-5 Linearity FD Special Diabetes product. Please see the attached sell sheet. This product should already be in your system.
If you have any questions, please let me know.
Have a great day!
Communication Type:
Product Flyer
V#12466
-
12/18/2024
Title: Sempermed V#10078
Details:
Companyc. Name Change to HARPS USA, In
December 13, 2024
Following our acquisition by HARPS Global on September 1, 2023, we would like to inform you that we have now changed our company name from Sempermed USA, Inc. to HARPS USA, Inc.
Please note that we have made no other changes. Our company address, phone numbers, email addresses, tax number, and bank account details will remain unchanged.
Over time, you will notice the transition to our new name, HARPS USA, Inc., on product packaging, marketing materials, websites, shipping documents, communications, etc.
We look forward to continuing our valued relationship under our new name, HARPS USA, Inc. If you have any questions, please contact us at 800.366.9545 or gloveinfo@harpsglobal.com.
Please update your records accordingly.
Copyright (C) 2024 HARPS USA, Inc.. All rights reserved.
You are receiving this email because you have opted in to receive product/company updates.
Our mailing address is:
HARPS USA, Inc. 13900 49th St N Clearwater, FL 33762 USA
Want to change how you receive these emails?
You can update your preferences or unsubscribeCommunication Type:
Updated Supplier Information
V#10078
-
12/17/2024
Title: Cardinal V#10801
Details:
upcoming change to a facility address.
Dear Valued Supplier,
This communication is meant to inform you of an upcoming change to a facility address.
Cardinal Health has embarked on a multi-year strategy to expand our U.S. warehouse capacity and modernize our medical distribution footprint. As part of this strategy, our new, nearly 249,000 square foot facility in Walton Hills, Ohio, is tentatively scheduled to begin operations February 23, 2025. The new Walton Hills distribution center will integrate new technology solutions, deliver operational efficiencies, and provide expanded capacity in the greater Cleveland area.
The additional capacity in our Walton Hills location provides an opportunity to both maximize and simplify our Cardinal Health operations. As such, we will be consolidating operations for Solon customers and closing the Solon facility February 20, 2025, and servicing these customers out of Walton Hills moving forward.
Important Next Steps
To ensure a successful transition, please make the following changes to your system effective for any shipments scheduled to arrive after February 20, 2025.
D029
Old Distribution Center
New Distribution Center
Name
Solon
Walton Hills
Address
5260 Naiman Parkway
Solon, OH 44139
22000 Alexander Road
Walton Hills, Ohio 44146
- Begin shipping to the Walton Hills facility any loads that will arrive on February 20 or after. If requesting an appointment close to this date, the Solon Inbound Team will confirm destination.
- Any shipments sent to the Solon location after this date will be redirected to Walton Hills.
- If you are utilizing an EDI interchange service, work with your EDI provider to determine if a boarding request will be required to update the facility address.
- License copies are attached for your records.
The Plant ID of D029 will not change due to this transition.
Please reach out to your Buyer (email address beginning with GMB-MRP3xx) with any questions.
Additionally, our National Brand Supplier Guidebook is attached to this communication as a point of reference for contact information or FAQs on doing business with Cardinal Health.
Thank you for your continued support of our mutual customers.
Cardinal Health Medical Distribution
Communication Type:
Updated Supplier Information
V#10801
-
12/17/2024
Title: Rockwell V#10934
Details:

PRODUCT LABELING (COLOR) CHANGES
Effective January 6, 2025Dear Valued Customer,
Reminder! Effective Monday, January 6, 2025, Rockwell Medical will be changing the color of its acetic acid (liquid and dry), citric acid (liquid and dry), and bicarbonate (liquid and dry) hemodialysis concentrates product labeling. This color change is part of our ongoing commitment to elevate the overall customer experience and align to industry standards and best-practices.
New labels will be used on packaging starting January 6th. Within one week's time (starting the week of January 13th), you can expect to start receiving products with the new labeling. It is important to note that while the color of the labels will be different, the quality, integrity and formulations of our products will remain the same.
For your reference, here is a link to a 1-page overview :: Rockwell Medical Labeling Change Information :: that provides a before-and-after comparison of our acetic acid, citric acid, and bicarbonate labels. No other Rockwell Medical product labels will be impacted.

During this transition, it is possible that your clinic may receive a mix of current and newly labeled product (drums, cases, bottles, and/or bags) on a single pallet. Please note that we will work closely with you to minimize confusion and ensure a seamless transition. Should you need sample copies of our new labels, please complete this New Label Request Form.
If you have any questions or need further information, please feel free to contact our customer care team at custserv@rockwellmed.com.
Thank you for your continued trust in Rockwell Medical for your hemodialysis concentrates needs.
Sincerely,
Tim Chole
Chief Commercial Officer
Rockwell Medical, Inc.
Communication Type:
Product Changes
V#10934
-
12/17/2024
Title: Medline V#10605
Details:
Allocation Issues/ Medline/ BD/ Cardinal
Your account has allocation for one or more of our needles skus.
Due to BD and Cardinal disruptions we expect to take on heavy backorders for the next 2-3 months.
Below are sub options that we can offer until we re-stabilize early next year.

Communication Type:
Supply Disruption
V#10605
Communication Type:
Allocation Notification/Update
V#10605
-
12/16/2024
Title: ICU V#10642
Details:
EOL Oxygen and Medication Delivery Items Discontinued from ICU Medical.
Please see items below that will be discontinued on 12/31/24. Please click here for official letter from ICU. You can find your customer listing by selecting this link. Please reach out to Heather Burkett with any questions. Thanks,
C# (1) C# (2) Product Code Description UOM GTIN Hospital List Dealer List 217597 183267 1121 OXYGEN MASK MEDIUM CONCENTRATION, ADL, 7' SUPPLY TUBING 25/CA 25/CA 35019315105207 $49.25 $41.00 1123 OXYGEN MASK HIGH CONCENTR. 7FT. TUBING REBREATHER ADULT 25/CA 25/CA 35019315105269 $56.50 $47.00 217603 1126 AEROSOL MASK VINYL ADULT 50/CA 50/CA 35019315105221 $41.00 $34.00 792727 1131 OXYGEN MASK ADULT 1L BAG, 7FT TUBING 25/CA 25/CA 35019315105276 $41.75 $34.75 217600 1171 OXYGEN MASK MEDIUM CONCENTR. WITH 7FT TUBING PEDIATRIC 50/CA 50/CA 35019315105214 $71.50 $59.50 792304 1176 AEROSOL MASK VINYL PAEDIATRIC 50/CA 50/CA 35019315105238 $59.50 $49.50 792307 1190 FACE TENT ADULT 40/CA 40/CA 35019315105245 $60.40 $50.40 1192 FACE TENT ADULT 25/CA 25/CA 35019315105252 $68.50 $57.00 216723 1203 OXYGEN TUBING, 7FT VINYL TIPPED 50/CA 50/CA 35019315105283 $28.00 $23.50 243745 1260 PED. NASAL CANNULA, SOFFLEX, 7FT TUBE, CURVED, NS + 50/CA 50/CA 35019315105153 $81.50 $68.00 221096 1261 NASL CAN SF NEO, 7FT TUBE 50/CA 50/CA 35019315105160 $95.50 $79.50 141331 1272 DRAINAGE BAG Y 50/CA 50/CA 35019315105344 $109.00 $91.00 1274 DRAINAGE BAG T 50/CA 50/CA 35019315105351 $86.00 $71.50 1283 NASL CAN SF AD C, 7FT TUBE 50/CA 50/CA 35019315105177 $48.50 $40.50 1285 NASAL CANNULA, 7 FT TUBE, STR FLARED, NS + 50/CA 50/CA 35019315105184 $57.50 $48.00 1289 NASAL CANNULA, 7 FT TUBE, CURVED FLARED, 50/CA 50/CA 35019315105191 $39.50 $33.00 217593 1290 NASAL ETCO2 OXYGEN CANNULA MALE LUER CONN., 7FT TUBING 50/CA 50/CA 35019315000014 $215.00 $179.00 216534 1291 NASL CAN OX F ADP 7, 50/CA 50/CA 35019315103463 $216.50 $180.50 216764 1292 NASL CAN OX M GL ADP 7, 50/CA 50/CA 35019315103470 $239.50 $199.50 1293 NASL CAN OX F GL ADP 7, 50/CA 50/CA 35019315103487 $239.50 $199.50 221095 1305 TRACH MASK ADULT 50/CA 50/CA 35019315105320 $195.50 $163.00 1356 TUBING OXYGEN 25/CA 25/CA 35019315105290 $51.25 $42.75 875963 1860 AEROSOL TUBING 6FT LENGTH 50/CA 50/CA 30351688406846 $86.00 $71.50 1861 AEROSOL TUBING 5FT LENGTH 50/CA 50/CA 30351688411949 $69.50 $58.00 Communication Type:
Discontinuation Notice
V#10642
-
12/16/2024
Title: Fresenius V#13576
Details:
Product Code Changes from Fresenius Kabi- Please see below from Item Maintenance and link below from FK.
Please click here for official Fresenius Kabi PDF.
I have added the last two to item inactivation and set up the last two new codes on the attached list for Fresenius-Kabi.
CHS#393523 mfg#624650 Disc by mfg-Sub is CHS#408346 mfg#416460 – no usage copied since this a sub.
CHS#393524 mfg#624661 Disc by mfg-Sub is CHS#408349 mfg#416450 – no usage copied since this a sub.
Already addressed in the system
CHS#393525 mfg#624674 Already addressed and directed to CHS#407547 mfg#416430
CHS#393526 mfg#624675 Already addressed and directed to CHS#407488 mfg#416420
Bottom half of the attachment we in IM only have the new codes set up.
CHS#393515 mfg#416630
CHS#393510 mfg#416620
CHS#393431 mfg#416610
Please let me know if you should have any questions.
Communication Type:
Product Changes
V#13576
-
12/12/2024
Title: Aspen Surgical V#10234
Details:
Aspen Surgical has made the decision to discontinue our beard cover offering (SKU 35-3015). It looks like over the last ~15 months, Concordance has purchased $6,433 worth of this SKU. Attached is our formal discontinuation notice.
I’m copying Tyler Farmer and our Product Manager Kushal Basnet. Please let us know if you have any questions or need anything else.
Thanks,
Kurt Kapfer
VP, NON-HOSPITAL SALES & CHANNEL PARTNERS
kurt.kapfer@aspensurgical.com
MOBILE / 1-847-421-3302
CUSTOMER SERVICE / +1 888.364.7004
FAX / +1 888.364.5381
6945 Southbelt Dr. SE, Caledonia, Michigan 49316 USA
aspensurgical.com
Aspen™ Bard-Parker® Bookwalter® Bovie® Olsen® Precept® Symmetry®
Communication Type:
Discontinuation Notice
V#10234
-
12/12/2024
Title: Abbott Nutrition V#10575
Details:
We are sharing with all of our distributor partners the attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Joe
Joe Corey
Sr. Business Analyst
Abbott Nutrition
Commercial Operations
Abbott
3300 Stelzer Rd.
RP 2-3
Columbus, OH 43219
O:
+1 614-624-6600
F:
+1 614-727-3896
Communication Type:
Product Inventory Update
V#10575
-
12/12/2024
Title: Integra Lifesciences V#10275
Details:
These are orders for the MediHoney HCS dressing that we show open with Concordance and would like to have substituted with the recommended MediHoney Hydrogel dressing due to supply challenges. A customer letter is attached for reference. The buyers for these PO’s can either call/email our CS team to cancel these PO’s and resubmit new PO’s or simply revise the already existing PO to reflect the Hydrogel sku of choice if choosing to go with the Hydrogel dressing and send into the CS team to ensure the change to the order is reflected correctly and updated for shipping same day.
I am also attaching a piece of literature for the MediHoney Hydrogel dressing in case helpful. The ONLY difference between the HCS and the Hydrogel dressings are that the Hydrogel dressings offer reimbursement.
Hydrogel sku’s are 31620, 31640, 31720 and 31740. You will see these listed on both the customer letter and the backside of the sell sheet attached.
Mary, of course if you have any questions or concerns please never hesitate in reaching out to me at any time. I apologize for any inconvenience but absolutely appreciate your support. Wishing you a fantastic start to the new week!
Warm regards,
Steph
Stephanie Williams
Associate Director, Distributor Relations
Tissue Technologies
609.936.4616 office • 609-213-9131 cell • 609.750.4277 fax
stephanie.williams@integralife.com
Integra • 1100 Campus Road, Princeton, NJ 08540
www.integralife.comCommunication Type:
Discontinuation Notice
V#10275
-
12/12/2024
Title: Attends V #10055
Details:
--NEW-- 2025 Attends Catalog (44832)
Concordance Team,
Please see the new 2025 Attends catalog that is attached.
Communication Type:
Product Catalog
V #10055
-
12/11/2024
Title: Welch Allyn V #10834
Details:
BP Cuff Supply Update_Customer Communication_Dec10_2024
Concordance Team,
There are no updates to allocations for BP cuffs this week.
- The allocation updates made last week are anticipated to remain until sometime in January when Welch Allyn advises that new allocation amounts will take effect.
- Production will continue to run 24/7 with the exception of December 25th.
- Further draw down of backlog in Q1 2025 will occur when new capital equipment is installed to increase production volume.
- The supplier recently created a webpage that has been updated effective today (12.10.24) Blood Pressure Cuff Supply Updates | Baxter
Communication Type:
Allocation Notification/Update
V #10834
-
12/9/2024
Title: Baxter V #10797
Details:
Baxter allocation 11.25.24 Customer Listing
Concordance Team,
Baxter issued a new customer communication letter before the holiday in regards to IV Products.
- They increased allocation levels per the attached.
- Allocations have been updated in our system and a customer listing is attached.
- Per the letter, item number UE1323 is a product that Baxter has available and sources outside the U.S. and is a substitute for current item 2B1323Q.
- Per the letter, item number A6C1322US is a product that Baxter has available and is temporarily sourcing outside of the U.S. and is a substitute for current item 2B1322Q.
Thank you.
Communication Type:
Allocation Notification/Update
V #10797
-
12/9/2024
Title: Nestle V#10183
Details:
If you have any questions about the product updates contained in this email, please contact your NHSc Distributor Manager.
Have a great week!
Amy Sauber, RDN, LDN
Manager, Sales Support Team
Nestlé Health Science U.S.
CONFIDENTIALITY NOTIFICATION: This email message and any attached documents are intended only for the use of the intended recipient(s) and may contain information that is confidential. If you have received this email in error, please notify the above sender and delete it from your computer.
Communication Type:
Product Inventory Update
V#10183
-
12/9/2024
Title: Nestle V#10183
Details:
Here’s the Nestle Inventory report from Friday
- Boost Kids Essentials vanilla was added
- Compleat Pediatric Peptide 1.5 was added
- Nutren Junior Fiber was added
- Impact Peptide 1.5 tetras and ultrapack are still OOS
All other products were removed, they are now back in stock
Glen Lundquist
Senior Manager National Accounts-Distribution
Nestle HealthCare Nutrition
Cell Phone: 847-601-5530
Email : glen.lundquist@us.nestle.com
Experience our Support Site
Communication Type:
Product Inventory Update
V#10183
-
12/9/2024
Title: Molnlycke V #10008
Details:
Molnlycke Healthcare 2024 Year-End Shut Down
Concordance Team,
Molnlycke will be closed on December 24th and 25th 2024, as well as on January 1st, 2025. They request that all normal weekly order volumes for December 23rd through January 1st be placed between December 9th and December 20th and to please allow an additional 3 days to your expected delivery date due to additional processing time based on the volume increase.
Please see attached notification.
Thank you.Communication Type:
Holiday Schedule
V #10008
-
12/8/2024
Title: Medtronic V#10255
Details:
Medtronic Med Surg Progrip Mesh Recall Product Lot Number List
Medtronic Med Surg Recall - Customer Confirmation Form Progrip Mesh RE00539461_EN_A_Appendix B
Medtronic Med Surg Recall Notification ProGrip Mesh RE00539461_EN_A_Appendix A
Vendor Item List Sales History (2 recalled items)_usage 5.6.24+7 months
Concordance Team,Medtronic is voluntarily recalling two specific lot numbers of Progrip™ Self-Gripping Polyester Mesh. This voluntary recall is being conducted due to incorrect device size being contained in the package. The two affected CHS item numbers are 154042 and 167880. Details related to this are provided in the attachments. Note: First ship date July 25, 2024 through last ship date November 4, 2024.
All of this has been sent to the CHS recall coordinator.
Thank you.
Communication Type:
Recall Notification
V #10255
-
12/8/2024
Title: Welch Allyn V #10834
Details:
BP Cuff Supply Update_Customer Communication_Nov15_2024
1_Copy of WA ALLOC2 % CHANGE 11-25-24
2_Vendor Item List Sales History sales usage 5.3.24-12.2.24
3_FlexiPort Configured Cuffs to Trimline Cross
5_TEMP UNAV CUFFS- Customer Listing
Concordance Team,
Allocations for the Welch Allyn BP cuffs were updated last week.
- 1st Excel file – from Casey which shows the updated allocation per item (either 40% or 100%).
- 2nd Excel file - usage for the past six months.
- 3rd, 4th and 5th Excel files – from Casey which are for the following scenario:
- Welch Allyn’s production continues to run at 100% capacity, but they needed to increase production of FlexiPort RAW cuffs, which have the highest demand and backlog.
- To prioritize increased production volume of FlexiPort RAW cuffs, they temporarily suspended production of FlexiPort Configured Cuffs.
- This will only affect Disposable Flexiport Cuffs and there are substitute items available.
- Flexiport Configured Cuffs include the cuff with tubing (1 tube or 2 tube) and have the various connectors (bayonet, luer lock, bulb/valve, etc.) while the Flexiport RAW Cuff is just the cuff with no tube or connectors shipped with it.
- This decision is founded in the fact that customers using FlexiPort Configured Cuffs have substitute options that RAW customers do not.
- For Trimline substitute options for Flexiport Disposable Configured cuffs, please reference the attached cross-reference guide (“FlexiPort Configured Cuffs to Trimline Cross”)
- Some Trimline items are already set up in VAI.
- For Customers moving from FlexiPort Fully-Configured to Trimline, Welch Allyn stated they can support the new demand on the Trimline cuffs.
Thank you.
Communication Type:
Allocation Notification/Update
V #10834
-
12/8/2024
Title: J&J V #10196
Details:
Biosurgery Customer Communication_Surgifoam_Supply Disruption_Nov 25 2024_FINAL
J&J Ethicon_6 items_sales 5.27.24-11.27.24
Concordance Team,
In the attached letter, J&J V #10196 provided an update about supply disruption for SURGIFOAM codes.
- The increase in volume and quantity of orders for SURGIFOAM codes has continued to result in unexpected shipping delays.
- They estimate returning to normalized supply in Q1 2025.
- Impacted mfr codes are 1972, 1973, 1974, 1975, 1977, 1978.
- The customer listing is attached which includes the cross to Concordance item numbers.
- These have been put on allocation.
Please refer to the supplier’s letter for more information, including a contact phone number if needed.
Thank you.
Communication Type:
Allocation Notification/Update
V #10196
-
Communication Type:
New Product Launch
V#10987
-
12/6/2024
Title: MicroCare V#10201
Details:
Rebranding
Dear valued partner,
I want to let you know that we have begun our rebranding effort.
Our ProEZ Foam is now available as Spec Clean Foam.
The only changes that were made were to the name and imagery.
The formula did not change nor did the item number.
I’ve attached our new Technical Data Sheet and our product change notification letters.
If you receive any feedback from customers, please let them know that the product is the same as it’s always been. We’ve just updated the name and image.
Feel free to contact me with any questions.
Have a great weekend!
Charlie Campbell
Key Account Manager
Microcare LLC | 6120 East 58th Avenue Commerce City, CO 80022
Email: CharlieCampbell@MicroCare.com| Mobile: 720.812.2432
www.Microcare.com/certol | www.ACIDMagic.com
Communication Type:
Product Update
V#10201
-
12/5/2024
Title: CryoConcepts V#12777
Details:
providing you the links to our Histofreezer FLEX training videos. See all links below. Can we get these posted to your Hubspot CRM?
Histofreezer FLEX - What's In The Kit? - YouTube
Histofreezer FLEX - How to Use Buds - YouTube
Histo Foam Buds Explainer Video
Histofreezer FLEX - How to Use Cones - YouTube
Histofreezer FLEX - How to Use Cones from Other Portable Cryosurgical Kits - YouTube
Histofreezer FLEX - How to Return An Empty Canister - YouTube
Product Website: HistoFreezer Flex | Skin Tag Freezer | CryoConcepts
Douglas W. Gavin
Regional Sales Director, East
CryoConcepts
M: 201-543-8270
O: 215-929-8659
Communication Type:
Product Flyer
V#12777
-
12/5/2024
Title: Abbott Nutrtion V#10575
Details:
We are sharing with all of our distributor partners the attached Abbott Nutrition inventory update.
Attached you will find the standard weekly inventory update letter you have been receiving:
- Abbott Institutional Inventory Letter
- Customer File Concordance
When product demand outpaces supply, we may need to allocate certain Adult Oral Nutrition Supplement SKUs, and currently we are proactively managing orders to deliver products to as many patients as possible. These product allocations will be based on historical demand of your acute care customers. Accordingly, Abbott requests you make these Adult Oral Nutritional Supplement product allocations initially available to fulfill your acute care customers’ demand.
For these product allocations you will be notified separately of these allocations. When your order quantities exceed the product allocations, these items will be cut from your orders.
If you have any questions, please contact your Distributor Relations team member from the list below.
Distributor Relations Contact Information:
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Judi Troutman; Manager: email: judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Joe
Joe Corey
Sr. Business Analyst
Abbott Nutrition
Commercial Operations
Abbott
3300 Stelzer Rd.
RP 2-3
Columbus, OH 43219
O:
+1 614-624-6600
F:
+1 614-727-3896
This communication may contain information that is proprietary, confidential, or exempt from disclosure. If you are not the intended recipient, please note that any other dissemination, distribution, use or copying of this communication is strictly prohibited. Anyone who receives this message in error should notify the sender immediately by telephone or by return e-mail and delete it from their computer.
Communication Type:
Product Inventory Update
V#10575
-
Communication Type:
Holiday Schedule
V#10262
-
Communication Type:
Product Update
V#12125
-
Communication Type:
Holiday Schedule
Vendor #11003
-
12/2/2024
Title: Roche V#10603 - Holiday schedule
Details:
FW_ Important Reminder_ Place Orders Online This Holiday Season!
Concordance Team,
Roche Diagnostics provided their list of days that they will be closed for the holidays and sent a reminder to place orders online and early:
We wanted to send you a friendly reminder to place your orders online! This time of year tends to get really busy, and we recommend ordering early to allow enough time for shipping. Please note that Roche will be closed on the following dates:
- November 28th-29th
- December 24th-25th
- December 31st
- January 1st
However, you can still place orders online, and our webshop will provide you with an estimated delivery date. Sign in to your Roche Navify account here: navifyportal.roche.com.
Communication Type:
Holiday Schedule
Vendor #10603
-
Communication Type:
Holiday Schedule
Vendor #12264
-
12/2/2024
Title: Kenvue V #10860
Details:
FW_ Important Reminder_ Place Orders Online This Holiday Season!
Concordance Team,
The Kenvue Brands supplier V #10860 shared their holiday schedule:
Please have all orders for December delivery entered no later than Monday, December 16th. Kenvue DCs will shut down December 24th & 25th. If you require deliveries the first week of January, please have all orders placed no later than Friday, December 20th. Kenvue DCs will be closed Tuesday December 31st and Wednesday January 1st.
Thank you,
Communication Type:
Holiday Schedule
Vendor #10860
-
12/2/2024
Title: Stryker Instruments V #10912
Details:
Stryker 10912_3 product disco usage May 13-Nov 13 2024
Concordance Team,
Stryker announced the discontinuation of the following items as of November 20, 2024. Their letter and our six-month usage are attached.
The disposable part numbers listed below will be obsolete as of November 30th, 2024.
5400-701-000 3.2mm Fine Blade
5400-702-000 5.0mm Medium Blade
5400-703-000 8.0mm Coarse Blade
As a replacement, we can offer our next generation, the Bone Mill +, with new Prep + disposable that removes soft tissue from appropriately sized bone pieces.
5420-100-000 Bone Mill + Base
5420-FNE-000 Mill + Fine Blade
5420-MED-000 Mill + Medium Blade
5420-CRS-000 Mill + Coarse Blade
5420-PRP-000 Prep + CartridgeKind regards,
Communication Type:
Discontinuation Notice
Vendor #10912
-
12/2/2024
Title: Health O Meter V #10075
Details:
RE_ HOM December Highlight to Sales Team
Concordance Team,
Health O Meter V #10075 has shared a new video on Accuracy Comparison between their Patient Transfer Scale PTS-1000KL (CHS # 323620), a Typical ICU Bed, and a Typical Stretcher. They stress that their PTS product, in comparison to the traditional bed and stretcher scales, stands out for its accuracy, which provides a safe and reliable solution for obtaining quick and accurate patient weights. The link to the video can be found in the attached email. Watch PTS Accuracy Comparison Video
Regards,
Communication Type:
Product Update
Vendor #10075
-
11/30/2024
Title: J&J V#10196 - Supply Disruption
Details:
Biosurgery Customer Communication_Surgifoam_Supply Disruption_Nov 25 2024_FINAL
J&J Ethicon_6 items_sales usage 5.27.24-11.27.24
Concordance Team,
In the attached letter, J&J V #10196 has provided an update about supply disruption for SURGIFOAM codes.
- The increase in volume and quantity of orders for SURGIFOAM codes has continued to result in unexpected shipping delays.
- They estimate returning to normalized supply in Q1 2025.
- Impacted mfr codes are 1972, 1973, 1974, 1975, 1977, 1978.
- The customer listing is attached which includes the cross to Concordance item numbers.
- The letter states that distributors (Concordance) should continue to place orders.
Please refer to the supplier’s letter for more information, including a contact phone number if needed.
Communication Type:
Supply Disruption
Vendor #10196
-
11/27/2024
Title: BD V#10125 - Holiday Schedule
Details:
2024 Holiday Schedule_FINAL_11-8-24
Concordance Team,
To help effectively plan your order fulfillment needs, enclosed is a copy of BD’s Q4 2024 Holiday Schedule.
*Please note the following:
- BD will be fully closed in observance of the Thanksgiving Day Holiday on Thursday, 11/28. There will be no Customer Care coverage available. Neither rush orders nor deliveries will be processed.
- BD corporate offices will be closed on Friday, 11/29. There will be no Customer Care coverage available. No rush orders will be processed.
With any further questions you may have, please reach out to Heather Burkett.
Kind Regards
Communication Type:
Holiday Schedule
Vendor #10125
-
11/24/2024
Title: BD V#10125
Details:
BD Secondary Set- MS3500-15 Discontinuation
Please see official Notice here.
BD code MS3500-15 has been depleted.
Orders will begin being cancelled by Evan Martini in the BU on Dec 2nd. All open orders will also need to be canceled.
RE: Supply update for BD Secondary Infusion Set; BD#MS3500-15
Dear Valued Partner:
On July 10, 2024, BD had notified customers that the BD Secondary Infusion Set; BD#MS3500-15 would be discontinued and provided BD Secondary Infusion Set; BD#10016073 as an alternative. Customers were also notified that production of the discontinued set may continue until December 31, 2024, with remaining product available until inventory is depleted.
Product Description
Alternate Catalog Number
Alternate Product Description
Product Differences
MS3500-15
SEC SET 36” 15DRP UNVTD W/HNGR ROLL CLMP SPIN LL
10016073
SEC SET 31” 20DRP V/NV W/HANGER ROLL CLMP SPIN LL
20 drop instead of 15 drop
5 in. shorter in length
2 ml lower priming volume
Communication Type:
Discontinuation Notice
Vendor #10125
-
11/22/2024
Title: Cardinal V#10801
Details:
Dear Valued Distributor,
On November 21, 2024, our Riverside, Calif. replenishment center (RC) experienced a third-party warehouse management system (WMS) outage (Blue Yonder) which has impacted operations.
Until the third-party issue is resolved, alternative RCs in our network will be used to ship and receive products which may result in a delay to your product deliveries. The RC teams will continue to work around the clock as well as weekends to resolve the backlog and help ensure our customers receive the product they need as quickly as possible.
Thank you for your patience and understanding. We will provide regular updates as they are available.
Please contact your account manager if you have any questions.
Channel Partners Group
Communication Type:
Supply Disruption
Vendor #10801
-
11/22/2024
Title: HollisterV#10901
Details:
Hollister update new product launches
- This is a product enhancement, no change to SKU’s, dimensions, weights, etc. just will offer consumers a better and more comfortable cathing experience
- These are very low-moving SKU’s, but here’s Concordance sales-out volume through Sept by affected SKU. These will likely be out of inventory end of Jan, early Feb timeframe, and I’ll update you as we get closer to depletion.
14206
88401
7740
Concordance
$ 4,212
$ 0
$ 0
Communication Type:
Discontinuation Notice
Vendor #10901
Communication Type:
New Product Launch
Vendor #10901
-
11/21/2024
Title: Integra Lifesciences V#10439
Details:
MediHoney HCS dressing that we show open with Concordance and would like to have substituted with the recommended MediHoney Hydrogel dressing due to supply challenges. A customer letter is attached for reference. The buyers for these PO’s can either call/email our CS team to cancel these PO’s and resubmit new PO’s or simply revise the already existing PO to reflect the Hydrogel sku of choice if choosing to go with the Hydrogel dressing and send into the CS team to ensure the change to the order is reflected correctly and updated for shipping same day.
I am also attaching a piece of literature for the MediHoney Hydrogel dressing in case helpful. The ONLY difference between the HCS and the Hydrogel dressings are that the Hydrogel dressings offer reimbursement.
Hydrogel sku’s are 31620, 31640, 31720 and 31740. You will see these listed on both the customer letter and the backside of the sell sheet attached.
Mary, of course if you have any questions or concerns please never hesitate in reaching out to me at any time. I apologize for any inconvenience but absolutely appreciate your support. Wishing you a fantastic start to the new week!
Warm regards,
Steph
Stephanie Williams
Associate Director, Distributor Relations
Tissue Technologies
609.936.4616 office • 609-213-9131 cell • 609.750.4277 fax
stephanie.williams@integralife.com
Integra • 1100 Campus Road, Princeton, NJ 08540
www.integralife.comCommunication Type:
Temp Unavailable
Vendor #10439
-
Communication Type:
New Product Launch
Vendor #10987
-
11/19/2024
Title: Airlife V#11179 - Product Discontinuation Notices
Details:
WESTMED ETCO2 DISCONTINUATION (002)
VYAIRE ETCO2 DISCONTINUATION (005)
11-19-2024 Westmed & Vyaire ETCO2 Product Discontinuation Notice Item Usage
11-19-2024 Westmed & Vyaire ETCO2 Product Discontinuation Notice Item Usage
The following Vyaire/Westmed products have been discontinued and will be replaced with a Salter Labs option:
VYAIRE O2 DISCONTINUATION (002)
Vyaire O2 Product Discontinuation 3 CHS Cross Ref.
11-19-2024 Vyaire O2 Product Discontinuation Item Usage
Please find enclosed details regarding Vyaire 02. The last buy date would be 12/31/2024.
2K805x Product Discontinuation CHS Cross Ref.
11-19-2024 2K805x Series Product Discontinuation Item Usage
As part of our business process, AirLife routinely evaluates its product portfolio and occasionally discontinues the manufacturing and distribution of certain products and materials. AirLife acknowledges the obligation to promptly inform our customers of the intent to exit products to enable our customers to prepare for alternatives and assist in their product portfolio renewal process. This letter serves as a formal notification that AirLife has discontinued production of the product(s) found on ATTACHMENT A.
With any further questions, please reach out to Jeff Shook.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #11179
-
11/19/2024
Title: Graham Field V#10228
Details:
Graham Field Sales Sheet
Please see link regarding offer from Graham Field- Buy any Hausted ESC2 and get a pair of Shoulder Rail Extensions for $200 — over $150 in savings • Item No: HSA080013-049
Communication Type:
Rep Spiff
Vendor #10228
-
11/18/2024
Title: Mckesson - OTC V#12901 - Product Discontinuation
Details:
11-18-2024 Mckesson Product Discontinuation Item Usage
Concordance Team,
Please see the discontinuation notice from Item Maintenance and the information attached for further info regarding this Mckesson - OTC V#12901 product discontinuation notice.
CHS# 700072 has been placed into item inactivation and the replacement has been created under CHS#407847.
With any further questions, please reach out to Tricia Wentling.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #12901
-
Communication Type:
Product Flyer
Vendor #12777
-
Communication Type:
New Product Launch
V#10801
-
Communication Type:
Holiday Schedule
Vendor #10801
-
Communication Type:
Holiday Schedule
V#12219
-
11/15/2024
Title: Cardinal V#10801 - Product Discontinuation
Details:
Ultrasound Gels Discontinuation_Customer Letter 8.16.24
Ultrasound Gel Discontinuation Item Cross Ref.
09-18-2024 Ultrasound Gel Discontinuation Item Usage
Concordance Team,
Per the attachment, the following items are discontinued:
Mfg#USG-250BT is already inactive in our systemMfg#USG20-NS is not set up in the system
S#358115 MFg#USG20ST placed in item inactivation as discontinued and replacement MFg#USG20STA – not set up due to lack of sales
Mfg#USG-5L is already inactive in our system
With any further questions you may have, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10801
-
11/14/2024
Title: Abbott V#10575 - Weekly Nutrition Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 11-11-2024. Also attached is a usage report for the Low Inventory Adult Institutional Items from the last 6 months.
Please note: There was no usage information for the Adult Inst. Deprioritized and the PSN Deprioritized items from the last 6 months.
Please see the attachments listed below to reference this notification
- Abbott Institutional Inventory Letter 11_14_24
- Customer File Concordance Cross Ref. 11-14-24
- 11-15-2024 Abbott Weekly Inv. Update Item Usage
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
11/14/2024
Title: Medline V#10605 - Product Conversion Update
Details:
11-16-2024 Medline Product Conversion Item Usage
Concordance Team,
Please see the product conversion notice update from Medline regarding their Cath Grip items:
Hello Distributor partners,
You are currently using a Medline Co-labeled securement device that is being replaced by our new Medline TotalSecure device.
Cathgrip is a hydrocolloid based universal securement device. TotalSecure is still a universal securement device with a hydrocolloid base.
We have made some improvements by simplifying application steps, utilizing a 2-in-1 alcohol and prep wipe.
Starting in October 2024, all Non-sterile CathGrip sku’s will be converting to the new TotalSecure device.
Please refer to the chart to note sku’s that need to be changed to an updated sku #.
All sterile sku’s will only be available in non-sterile. If you wish to purchase the sterile items, please connect with your urology sales specialist for alternate options and timeline.


Thanks,
Mansi
With any further questions, please reach out to Heather Burkett.
Kind Regards
Communication Type:
Product Changes
Vendor #10605
Communication Type:
Product Update
Vendor #10605
-
11/13/2024
Title: NDC V#10030 - Product Discontinuation
Details:
Concordance Team,
Please note CHS#366940 (MFG#6745KG) has been placed into item inactivation and it should be fully inactivated as of 11/13/2024. The item has been directed to CHS#100225 (MFG#6745) for future sales. per Detecto.
With any further questions, please reach out to Mary Reinhart.
Kind RegardsCommunication Type:
Discontinuation Notice
V#10030
-
11/13/2024
Title: Welch Allyn V#10834 - Allocation Update
Details:
Welch Allyn BP Cuffs - Impacted Part Numbers (002)
Concordance Team,
Please see below and the attached for further information update on Welch Allyn’s Allocation Percentage increase to 40%.
With any further questions you might have, please reach out to Tricia Wentling.
Also, from the desk of Marianna Snavely:
Welch Allyn BP Cuffs - Impacted Part Numbers (002)
MC13190_Flexiport_CuffBrochure-WR
JB00412XX_CRITIKON-CLASSIC-CUF_Sell-Sheet_Non-CE_v7
DINACLICK Sell Sheet_Non-CE_Updated_2023_JB00467XX
Good Morning,
I reviewed the attached product list (1st attachment) and I spoke with Welch Allyn. There is not “technically” another manufacturer that makes a compatible blood pressure cuff to be used with the Welch Allyn / Baxter blood pressure machines. The raw material shortage, they are experiencing is effecting all of their blood pressure cuffs. So even though there are adaptors or different types of material cuffs, they said there is not any extra availability of those options to use as an alternate. Their suggestion was to clean the cuffs and suggested calling 1-800-535-6663, option 2 for cleaning recommendations. They did say the product lines were completely shut down. They have resumed shipping but they are not able to provide any date for recovery.
The 2nd attachment, is the product guide for the Welch Allyn cuffs. Page 7 provides product numbers for the vinyl, soft and reusable interchangeable product numbers and connectors.
The 3rd & 4th attachment is the GE Critikon BP cuffs. See text box below. Welch Allyn will not clinically support the use of the GE blood pressure cuffs but they are physically interchangeable. It would have to be a decision the customer makes if they are willing to use the GE cuffs. The 3rd and 4th attachment are the GE blood pressure cuffs with different materials and adaptors.

Please let Marianna Snavely & Tricia Wentling know if you have additional questions or I can help in anyway.
Kind Regards
Communication Type:
Allocation Notification/Update
Vendor #10834
-
11/13/2024
Title: Stryker V#10912 - Product Discontinuation Notice
Details:
Stryker 10912_3 product disco usage May 13-Nov 13 2024
Concordance Team,
Please see the discontinuation notice above for the 3 Stryker Items shown below. This is effective November 30th 2024.
Usage from the last 6 months is attached above as well.
Obsolete as of Nov 30
Obsolete Part# Desc
New Part#
New Part# Desc
5400-701-000
The Mill Disposable- Medium
5420-MED-000
THE MILL - MEDIUM
5400-702-000
The Mill Disposable- Fine
5420-FNE-000
THE MILL - FINE
5400-703-000
The Mill Disposable- Coarse
5420-CRS-000
THE MILL - COARSE
If you have any further questions, please reach out to Tricia Wentling.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10912
-
11/13/2024
Title: Solventum V#10296 - New Product Launch
Details:
attest-ebd-starter-kit-npi-digital-campaign-sell-sheet-ms-sa-en-pdfa
3M™ Attest™ eBowie-Dick Test System - Final Nov. 2024
Concordance_Supplier Item Attributes
Concordance Team,
Please see the attachments from Solventum V#10296 regarding their new product launch.
With any further questions, please reach out to Tricia Wentling.
Kind Regards
Communication Type:
New Product Launch
Vendor #10296
-
11/13/2024
Title: Medline V#10605 - Micro - Kill Lid Color Change
Details:
11-16-2024 Medline Product Color Change Notice Item Usage
Product Color Change CHS Cross Ref.
Concordance Team,
Please see the notice below from Medline V#10605 regarding the Micro-Kill Lid Color Change notice.
Dear Distributor partner,
This notice is to inform you of a packaging change for Medline Micro-Kill One Wipes. The product label for Micro-Kill One has changed colors from purple to pink. The scope of the change is to the product label only – no other product changes have occurred. The Micro-Kill One purple lid stock will be fully depleted by end of year, and all inventory will then have the updated pink lid.
Item Number
Previous color
New color
Purple
Pink
Micro-Kill One
MSC351300
MSC351310
Thank you,
Julie
With any further questions you may have, please reach out to Heather Burkett.
Kind Regards
Communication Type:
Product Catalog
Vendor #10605
-
11/12/2024
Title: Midmark V#10987 - Holiday Schedule
Details:
Concordance Team,
Please see the important 2024 End of Year Shipping Dates Update from Midmark below:
Dear Valued Partner,
In preparation for the holidays and year-end, we have updated our shipping schedule. Unless otherwise noted below, there will be normal shipping through December 23, 2024. Please take note of the following dates:
December 24-25, 2024 – No shipping: Closed for the holiday
December 26-27, 30-31, 2024 – Normal shipping
January 1, 2025 – No shipping: Closed for the holiday
January 2-3, 6, 2025 – Limited shipping due to inventory count: Shipping for “doctor down” parcel service shipments only
January 7, 2025 – Normal shipping resumes
Please contact the Midmark Medical Customer Experience Department at 1.844.856.1233, option 2, with questions.
Kind Regards
Communication Type:
Holiday Schedule
V#10987
-
11/12/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_8Nov2024
Nestle Weekly Inv. Update CHS Cross Ref.
11-11-2024 Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 11-11-2024. The following changes are being made:
- Vivonex RTF 1000 ml was added
- Peptamen Jr 1000 ml was added
- Isosource 1.5 1500 ml was moved
- Peptamen PreBio 1000 ml was removed
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
11/11/2024
Title: Abbott V#10575 - Product Availability Update
Details:
11-16-2024 Product Availability Update Item Usage
Concordance Team,
Abbott Nutrition is providing an update that the following SKUs will be available to order on 11/12/2024: These items will have quantities reviewed to ensure equitable access for patients across all channels.
MFG# VAI Item Item Description 68232 356393 Ensure Plus HP Strawberry 8oz ARC If you have any more questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Product Update
Vendor #10575
Communication Type:
Product Inventory Update
Vendor #10575
-
11/7/2024
Title: Sempermed V#10078 - Product Discontinuation Notice
Details:
11-07-2024 Product Discontinuation Item Usage
Concordance Team,
Sempermed has provided us with a list of their retiring gloves and the suggested subs.
The recommended sub for SUNB is SUNG which is their green glove. One could also replace SUNF with SMTN but there is a difference in packaging. SMTN has 250 to a box.
Neither SUNG or SMTN has the gastric acid claim that SUNF has.

If you have any further questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10078
-
11/7/2024
Title: Jant V#10420 - Product Flyer
Details:
Concordance Team,
Please see the product flyer from Jant V#10420 regarding their H. Pylori Urease Test.
With any further questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Product Flyer
Vendor #10420
-
11/7/2024
Title: Essity V#10272 - Product Change Announcement
Details:
65361 RN D1-F Pink Deviation Announcement
Concordance Team,
Essity is announcing a temporary change to their Delta-Cast casting tape line. They will be producing a new shade of pink affecting the following items:
Delta-Lite Plus
Delta-Lite Comfortable
Delta-Cast Elite
Delta-Cast Comfortable
Delta-Cast Soft
For more information, please see the announcement attached or reach out to Heather Burkett.
Kind Regards
Communication Type:
Product Changes
Vendor #10272
-
11/7/2024
Title: Medline V#10605 - Product Conversion
Details:
11-27-2024 Item Conversion Item Usage
Concordance Team,
Please see
DYND75029 (Tourniquet, Blue, 1”x18”, Rolled and Banded) converting to new SKU DYND75029A (Tourniquet, Blue, 1"X18",Folded and Banded).
Performance testing was evaluated on DYND75029A to compare the tensile strength and elongation against DYND75029. The results of the testing passed our acceptance criteria and demonstrated comparable behavior to DYND75029.
NEW Non-Formulary Customer SKU DYND75029A Formulary Customer SKU DYND75029 With any further questions, please reach out to Heather Burkett.
Kind Regards
Communication Type:
Product Changes
Vendor #10605
-
11/6/2024
Title: Felix Storch V#10121 - Product Discontinuation
Details:
Concordance Team,
CHS#262404 has been placed into item inactivation after speaking with CS Rep Raymond at Felix Storch.
- Per Jessica Day, Item Maintenance Specialist
Please note there is no usage in the last 6 months for CHS#262404.
With any further questions, please reach out to Tricia Wentling.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10121
-
11/4/2024
Title: Avanos V#11967
Details:
Product Discontinuation Letter:
I am writing to inform you about an important update regarding our CloTest and Jack Bean product lines. We will be discontinuing these products, and I wanted to ensure you are aware of this change well in advance.
The products in question will remain available for purchase until our inventory is exhausted, or until the end of January 2025, whichever comes first. After January 2025, any remaining open orders for these products will need to be removed from our system.
Please review the attached documents for detailed information on the discontinuation. Thank you for your understanding and cooperation.
If you have any questions please reach out to Heather Burkett. Thanks,
Please see added information from Jant: They are the recommended supplier for this in the Avanos attachment.
While a major manufacturer will be discontinuing their H. pylori Urease Test at the end of January, Jant Pharmacal is committed to filling the gap for rapid detection of H. pylori in gastric biopsies with the Accutest Rapid H.pylori Urease Test.
With 99% sensitivity and 97% specificity, this cost-effective, CLIA-waived test requires no reagents and provides positive results in as little as one minute and negative results in one hour.
The 50-test kit includes a control and can be stored at room temperature for 3 years.
Please contact us for additional information.
Communication Type:
Discontinuation Notice
Vendor #11967
-
11/4/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 10-28-2024. Also attached is a usage report for the Low Inventory Adult Institutional Items from the last 6 months.
Please note: There was no usage information for the Adult Inst. Deprioritized and the PSN Deprioritized items from the last 6 months.
Please see the attachments listed below to reference this notification
- Abbott Institutional Inventory Letter 10_31_24
- Weekly Inv. Update CHS Cross Ref.
- 11-05-2024 Weekly Inv. Update Item Usage
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
11/4/2024
Title: Cooper Surgical V#10052 - Silicone Diaphragm Discontinuation
Details:
Cooper Surgical Silicone Diaphragm discontinued Products
Concordance Team,
Please see the Silicone Diaphragm Discontinuation notice from Item Maintenance.
**Note: There was no usage for the affected products over the last 6 months.
We had 3 items set up in the attached list in VAI for the Cooper Surgical V#10052 Diaphragms. All 3 items fully inactivated with no replacement.
CHS#373712 mfg#MXWF70
CHS#335700 mfg#MXWF75
CHS#742948 mfg#MXWFFS
Thank you
If you have any further questions, please reach out to Tricia Wentling.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10052
-
11/4/2024
Title: Cooper Surgical V#10052 - Lugol's & AsrinGyn Discontinuation
Details:
Cooper Surgical Lugol's & AstrinGyn Discontinued Products
Concordance Team,
Please see the Lugol's & AsrinGyn Discontinuation notice from Item Maintenance.
**Note: There was no usage for the affected products over the last 6 months.
We had 2 items set up in the attached list in VAI for the Cooper Surgical V#10052 Lugol & AstrinGyn items. Both items fully inactivated with no replacement.
CHS#186662 mfg#6064
CHS#725167 mfg#6065
Thank you.
If you have any further questions, please reach out to Tricia Wentling.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10052
-
11/4/2024
Title: Welch Allyn V#10834 - Product Discontinuation
Details:
Discontinuation Notice from Welch Allyn - Baxter on Select Configurations
Concordance Team,
Please see the discontinuation notice from Welch Allyn regarding select Baxter configurations... this notice came from Jenn Pahl in Item Maintenance.
In the attached letter that was left on my desk , the following two items have been placed into item inactivation with recommended replacements.
CHS#246978 mfg#CP150AW-1ENB disc by mfg-see recommended replacements of CHS#209548 mfg#CP150A-1ENB or CHS#215453 mfg#BUR280-81X – no usage to copy.
And
CHS#265427 mfg#CP150AWD-1ENB disc by mfg-see recommended replacements of CHS#209548 mfg#CP150A-1ENB or CHS#315022 mfg#ELI289-AAA-AAFBD – no usage to copy.
Both items should inactivate in the overnight process tonight.
Please let me know if you should have any questions.
Thank you.
Please note there was no usage for the last 6 months for the effected product.
If you have any further questions, please reach out to Tricia Wentling.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10834
-
11/4/2024
Title: NDC V#10030 - Product Availability Update
Details:
Concordance Team,
NDC has notified us that WELLlife COVID-19 + Flu A/B Combo Test is now available. Please see below for further information:
WELLlife® COVID-19 + Flu A/B Combo Test Now Available!
Accurate, Rapid Differentiation of COVID-19, Flu A, and Flu B – Results in 10 MinutesStay prepared this flu season with the WELLlife® COVID-19 + Flu A/B Combo Test, available to NDC Distributors exclusively through Clarity Diagnostics. This innovative test delivers rapid and accurate differentiation between COVID-19, Influenza A, and Influenza B antigens—all from a single, cost-effective anterior nasal swab.
With results in just 10 minutes, the combo test is available in both over-the-counter (OTC) and point-of-care test formats, empowering faster, informed decisions for patients and providers alike.
By offering this exclusive combo test to your clients, you can meet the demand for efficient respiratory testing and enhance patient care.
To learn more, contact Clarity Diagnostics today at (877) 722-6339 or Sales@ClarityDiagnostics.com.
Please click the link below for the Clarity_WELLlife_COVID_19__Influenza_AB_POC_61224 pdf
Learn more about the WELLlife® COVID-19 / Influenza A&B Test >>
Learn more about the WELLlife® COVID-19 / Influenza A&B Home Test >>
Download the WELLlife® COVID-19 / Influenza A&B Test Quick Reference >>
Download the WELLlife® COVID-19 / Influenza A&B Test IFUs >>If you have any further questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Product Inventory Update
Vendor #10030
-
11/4/2024
Title: Hartmann V#10739 - Product Update
Details:
Customer Notification Letter-Omnifix Elastic 900608
11-06-2024 Hartmann Product Update Item Usage
Concordance Team,
Please see the attachments for your records.
Net Pricing Agreement NP-2409 Effective 8/1/2022 to 2/28/2025 – Product Update:
- Item 900608 – UOM Change from 24/CS to 36/CS.
If you have any further questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Product Update
Vendor #10739
-
11/4/2024
Title: Convatec V#10695 - Holiday Schedule
Details:
Concordance Team,
Please see below regarding the Convatec V#10695 holiday schedule/year-end orders update.

If you have any further questions, please reach out to Mary Reinhart.
Kind Regards,
Communication Type:
Holiday Schedule
Vendor #10695
-
11/4/2024
Title: Abbott V#10575 - New Product Launch
Details:
202420039_EleCare_Jr Update Customer Letter_10.24
New Product Launch CHS Cross Ref.
11-07-2024 New Product Launch Item Usage
Concordance Team,
Please see the New Item Launch 2025 regarding the EleCare Jr. Innovation from Abbott V#10575 below and attached.
Dear Distributor Partner –
Abbott is excited to announce that in the spring of 2025, we will replace our currently marketed EleCare Jr formula with an updated formula that has DHA and lutein. EleCare Jr will continue to be a nutritionally complete, hypoallergenic, well tolerated, amino acid-based formula to meet the needs of pediatric patients, will offer enhanced ingredients to support brain and eye development, and will feature the additional changes outlined in the attached customer letter.
These items are being added to your acquisition cost contract pricing as set forth in the table below:
Distributor Acquisition Cost (3)
Item
Current Item #
NEW Item #
New Item UPC (Case)
New Item UPC (Unit)
New Item NDC Format Code (Unit) (1)
Estimated Launch Timing (2)
1 - 124 Case Price
125+ Case Price
EleCare Jr Unflavored 14.1-oz can
55253
68625
70074686257
70074686288
70074-0686-28
April-25
$249.64
$239.64
EleCare Jr Vanilla 14.1-oz can
56585
68630
70074686301
70074686318
70074-0686-31
April-25
$249.64
$239.64
(1)NDC-format codes: NDC Format codes are product codes adjusted according to standard industry practice to meet the format requirements of pharmacy and health insurance computer systems. Abbott Nutrition does not represent these products to be drugs, nor their codes to be NDC codes. If used for third-party billing purposes, each healthcare provider or contracted vendor is ultimately responsible for the accuracy and appropriateness of codes billed for individual patients and services.
(2) Estimated launch timing is subject to change.
(3) 125 Cs Price / Case: All Abbott products have bracketed pricing based on the quantities ordered which is the price that is reflected on distributor's invoices from Abbott. For Contract Pricing (TN) the 125-case contract price is your Cost-Basis. The Cost-Basis price brackets are the cells outlined in red.
We recognize our customers and distributor partners play vital roles in making this a smooth and efficient transition. We recommend that customers maintain both current and new item numbers in their system for the duration of the transition. Additional information will be provided to keep you up to date on any new information regarding this important product transition.
The attached Customer letter is being distributed to our mutual end-user customers beginning this week with direction for them to work directly with their distributor representative on product availability for these items.
If you have any further questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
New Product Launch
Vendor #10575
-
11/4/2024
Title: Betco V#12185 - Holiday Schedule
Details:
Concordance Team,
Please see the link below for the holiday schedule for Betco V#12185.
2024 Betco Holiday Closing Schedule
With any further questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Holiday Schedule
Vendor #12185
-
11/4/2024
Title: GOJO V#10354 - Holiday Schedule
Details:
Concordance Team,
Please see the Holiday Schedule from GOJO V#10354.
GOJO Holiday Schedule Reminder – Q4 2024 and Q1 2025
As the holiday season approaches, we wanted to re-share the days we will be closed in observance of upcoming holidays. To facilitate on time delivery, please see additional lead time requested in the schedule below.

Below are key days to submit a Purchase Order to ensure shipment in 2024:
-
-
- December 6, 2024 – Order deadline for Truckload shipments (extra time to obtain delivery appt.)
- December 13, 2024 – Order deadline for Small Parcel and LTL shipments
- December 27, 2024– Last full shipping day in 2024
-
If you have any further questions, please reach out to Tricia Wentling.
Kind Regards
Communication Type:
Holiday Schedule
Vendor #10354
-
-
11/4/2024
Title: Nestle V#10183 - Holiday Schedule
Details:
Concordance Team,
Please see the attached holiday schedule from Nestle V#10183.
2024 Holiday Year-End Shipping Schedule 10-31-24
With any further questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Holiday Schedule
Vendor #10183
-
11/1/2024
Title: Essity V#10272
Details:
TENA Product Discontinuation Notice
Please use link to find notice from Essity on Tena products that will be discontinued on January 1st, 2025.
"To help better serve our customers, we will consolidate our lines: TENA Sensitive Care Light Pads, TENA Underpads, and TENA ProSkin Flex."
Usage will be copied on stock items and any active stock requests will be moved once stock is depleted since these are direct replacements.
If you have any questions feel free to reach out to Heather Burkett.
Communication Type:
Discontinuation Notice
Vendor #10272
-
11/1/2024
Title: Clarity Diagnostics V#12117
Details:
NEW 510(k) Cleared, CLIA-Waived, and Made-in-USA COVID antigen rapid test available from Clarity Diagnostics. Please see info below in links.
If you have any questions please reach out to Heather Burkett.
Communication Type:
New Product Launch
Vendor #12117
-
11/1/2024
Title: Hollister V#10901 - Device Recall Notice
Details:
Medical Device Recall_Customer Notification_CalciCare US all
Medical Device Recall CHS Cross Ref.
11-06-2024 Hollister Medical Device Recall Item Usage
Concordance Team,
See the below device recall from Hollister and attached for further information.
Hello,
In order to best serve the needs of our customers, Hollister Incorporated, the distributor, is forwarding a voluntarily recall from Advanced Medical Solutions, Ltd. (AMS), the manufacturer of CalciCare Calcium Alginate Dressing 4” x 4.75”. Our records indicate that you have received stock of the affected product, and you are therefore affected by this action.
We kindly request that you read the attached Device Recall Notice from Hollister and complete the actions listed in the document within 14 days of receipt of this Notice. Your firm will also be receiving the attached notification in the mail on Monday November 4, 2024.
Please reach out to ComplaintCommunication@Hollister.com with any additional questions or concerns. Thank you.
Best Regards,
Christine Ponka
Global Post Market Surveillance Manager
Hollister Incorporated
2000 Hollister Drive | Libertyville, IL | 60048
224-358-5642
www.hollister.comIf you have any further questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Recall Notification
Vendor #10901
-
10/31/2024
Title: Medline V#10605 - Product Discontinuation
Details:
10-31-2024 Product Inactivation Item Usage
Concordance Team,
***TUMBLER SIZE CHANGE FROM 10OZ TO 9OZ***, Medline kept the same mfg#***
CHS#225528 mfg#DYND80455 no longer making the 10oz size and have replaced it with a 9oz tumbler- See CHS#407306 mfg#DYND80455 – usage not copied due to stocking UOM being different.
CHS#332129 mfg#DYND80455 no longer making the 10oz size and have replaced it with a 9oz tumbler- See CHS#407306 mfg#DYND80455 – usage copied since stocking UOM are both at an EACH.
Please reach out to Jenn Pahl with any further questions you may have.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10605
-
10/31/2024
Title: Cardinal V#10801 - Product Discontinuation
Details:
222002A- Discontinuation Letter
10-30-2024 Cardinal Product Discontinuation Item Usage
Concordance Team,
Cardinal has informed us of the recent discontinuation of their MFG#222002A- (CHS#189544) reflective mylar neonatal electrode with aloe. The official discontinuation letter has been attached to share with their Channel 20 customers.

This discontinuation is unique. Demand on this item was relatively low compared to their other neonatal SKUs and they haven't been meeting th eminimum demand threshold for several months. Since the pland must order 3 years' worth of raw materials for 222002A- at a time, the projected return on investment wasn't high enough to continue the production of the product. Production on 222002A- has stopped due to the depletion of raw materials on-hand.
MFG#31424785 (CHS#116220) is the recommended alternative stated within the letter. This neonatal electrode is the same size and shape as 222002A-. The main difference is that it has a tape substrate and their standard hydrogel adhesive without the addition of aloe. Product details are below.

With any further questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10801
-
10/31/2024
Title: Abbott V#10575 - New Product Update
Details:
Abbott Oral Hydration Distributor Letter 10.23.24
Concordance Team,
Please see below and attached regarding product availability for hospital contracts.
If you need any of these SKUs set up in our system, please reach out to Item Maintenance.
For any further questions, please reach out to Mary Reinhart.
Kind Regards,
Communication Type:
New Product Launch
Vendor #10575
Communication Type:
Product Update
Vendor #10575
-
10/30/2024
Title: Cardinal V#10801 - Product Discontinuation Notice
Details:
222002A- Discontinuation Letter
10-30-2024 Cardinal Product Discontinuation Item Usage
Concordance Team,
Cardinal has informed us of the recent discontinuation of their MFG#222002A- (CHS#189544) reflective mylar neonatal electrode with aloe. The official discontinuation letter has been attached to share with their Channel 20 customers.

This discontinuation is unique. Demand on this item was relatively low compared to their other neonatal SKUs and they haven't been meeting th eminimum demand threshold for several months. Since the pland must order 3 years' worth of raw materials for 222002A- at a time, the projected return on investment wasn't high enough to continue the production of the product. Production on 222002A- has stopped due to the depletion of raw materials on-hand.
MFG#31424785 (CHS#116220) is the recommended alternative stated within the letter. This neonatal electrode is the same size and shape as 222002A-. The main difference is that it has a tape substrate and their standard hydrogel adhesive without the addition of aloe. Product details are below.

With any further questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10801
-
10/29/2024
Title: BSN V#10231
Details:
Product Discontinuation
Please see attached link for information on the discontinuation of C#194524 and C#195454 and suggested replacements.
If you have any questions please reach out to Heather Burkett.
Communication Type:
Discontinuation Notice
Vendor #10231
-
10/28/2024
Title: GOJO V#10354 - Intermittent Supply Disruption
Details:
GOJO UPDATE - CHG Product Supply Update - CONCORDANCE HEALTHCARE SOLUTIONS (100110)
Vendor Item List Sales History with Units_Apr1 2024 to Oct27 2024
Concordance Team,
Please see the attached and below for further information from GOJO V#10354 regarding an intermittent supply disruption affecting two SKUs.
A usage report was ran (Please see attached) for the effected items and only CHS#265614 had sales over the last 6 months.
8842-03 (C #265614) >> SEE GOJO’S SUB SUGGESTION BELOW:

5304-03 (C #160336) >> SEE GOJO’S TWO OPTIONS (INCLUDING SUB) BELOW:

Please reach out to Tricia Wentling with any further questions you may have.
Kind Regards
Communication Type:
Supply Disruption
Vendor #10354
-
10/28/2024
Title: J &J V#10196 - Product Discontinuation Notice
Details:
11-04-2024 Product Discontinuation Notice Item Usage
Concordance Team,
Please see below for an IMPORTANT notification from J&J (V #10196) about a temporary pause on orders until Jan 1, 2025, for three SKUs. The 4th item listed is being discontinued.
- Only item C #138610 (U202H) has a sub listed in VAI.
Mfg Stock Number
VAI Item
1915G
395968
K895H
645531
U202H
138610
U232H
146456
With any further questions, please reach out to Tricia Wentling.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10196
-
10/28/2024
Title: Baxter V#10797 - Product Expiration - Extended Dating
Details:
Customer Letter_HH_Expiration Date Extension_10.28.24
Concordance Team,
Baxter announced that the FDA has authorized EXTENDED use dates of specified product codes. Please refer to the attached letter for details
With any further questions you may have, please reach out to Tricia Wentling.
Kind Regards,
Communication Type:
Product Update
Vendor #10797
Communication Type:
Updated Supplier Information
Vendor #10797
-
10/28/2024
Title: O & M Halyard V#12264 - Holiday Schedule
Details:
Concordance Team,
Please see the Holiday Closure Alert from O & M Halyard V#12264.
October 28, 2024
Valued Customer,
We would like to inform you that Owens & Minor Distribution Centers will be closed on Thursday, November 28 and Friday, November 29, in observance of the Thanksgiving holiday.
Please note:
If you normally receive your orders on Thursdays or Fridays, unless previous arrangements have been made, all deliveries will be made on your next scheduled delivery day following the holiday. We ask that you adjust your orders accordingly.
Reach out to your Customer Service Team by end of day on Friday, November 15, 2024 to request alternative arrangements. The Customer Service Team will work with the shipping DC to determine if the request can be accommodated. Additional fees may be applied.
If no special delivery is requested and arranged, all freight and fees for any off-day deliveries requested will be applied.
Thank you for your partnership and have a safe holiday!
Sincerely,
Kumar Pushkal | Vice President, Distribution Operations
Owens & Minor | Products & Healthcare ServicesCommunication Type:
Holiday Schedule
Vendor #12264
-
10/28/2024
Title: Attends V#10055 - New Product
Details:
Attends Discreet Pads Flyer (2)
Attends Discreet Timeline 2024
Concordance Team,
The Attends bladder control pads made up of four (4) SKUs will have a “soft launch” of their improved product and new packaging.
See the item numbers in the supplier’s email below.
WHAT IS CHANGING:
- Their oracle number is changing as indicated by the word “New” in the item list (I added the Concordance numbers in red).
- The surface of the packaging is changing to be more aesthetically pleasing (that is it).
- The product performance was improved.
@^All Account Managers see the attached FLYER for full explanation to share with customers
WHAT IS NOT CHANGING:
- No changes to bag or case quantity.
- No changes to contract or acquisition pricing.
- No changes to packaging dimensions
As noted below from the supplier:
- We are updating and adding the items to all contracts which our contract team will send to yours once they are updated.
- Timeline & Attends Discreet Pad flyer (attached) you can share with your sales reps.
With this “soft launch”, the supplier noted during our call today that changes will be slow over the coming weeks.
- OPS: You may notice product arriving on pallets during this transition could sometimes include the old blue packaging + the new packaging.
- AMs: Make customers aware of the slow transition with the flyer.
- IM: Notice the link in the attached email for pulling new images.
Please use the link below to view Attends Digital Product Library.
https://app.salsify.com/catalogs/f2179af3-0941-4bbc-b336-a5f119f79a4f/products?filter=%3D&page=1
With any further questions, please reach out to Tricia Wentling.
Kind Regards
Communication Type:
New Product Launch
Vendor #10055
Communication Type:
Product Update
Vendor #10055
-
10/28/2024
Title: Abbott V#10575 - Product Availability Update
Details:
11-05-2024 Product Availability Update Item Usage
2Product Availability Update CHS Cross Ref
Concordance Team,
Abbott Nutrition is providing an update that the following SKUs will be available to order on 10/29/2024: These items will have quantities reviewed to ensure equitable access for patients across all channels.
MFG# VAI Item Item Description 68232 356393 Ensure Plus HP Strawberry 8oz ARC 64903 248670 Ensure Clear Apple 8oz ARC If you have any more questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Product Inventory Update
Vendor #10575
-
10/28/2024
Title: Baxter V#10797 - Product Update
Details:
Baxter Blood Pressue Cuff_Cleaning Communication_Oct2024
BP Cuff Supply Update_Customer Communication_Oct2024
Copy of Welch Allyn BP Cuffs - Impacted Part Numbers
Vendor Item List Sales History with Units Apr-Oct 2024
Concordance Team,
Please see the attached BP cuff update and customer listing/usage along with the allocation explaination from the supplier below
Thank you for your time. I wanted to connect with the team at Concordance regarding our Blood Pressure Cuff supply situation with Welch Allyn. As we discussed, we are experiencing a temporary constraint on raw material availability for our blood pressure cuff production out of our manufacturing plant in Mexico. U.S. imports of raw materials into Mexico are temporarily halted, pending release from the Mexican government authorities, which has led to a pause in production at our plant. We have provided all requested information to the authorities, and we understand the release to be imminent, but the exact timeline remains unclear. As soon as we receive the pending authorization, we will resume 24/7 production. This unplanned downtime will result in extended lead times, necessitating thoughtful conservation of supply, and implementation of a cleaning protocol for the BP cuffs as necessary. We are working expeditiously to bring supply back online as fast as possible.
Attached is the list of the impacted blood pressure part numbers. We would suggest putting these on a 20% supply allocation until we have further information on our ability to bring production back to 100%.
Also attached is the Customer communication we will be sharing directly with customers to encourage implementation of a cleaning protocol (also attached) on their current supply of blood pressure cuffs per the Instructions provided in the DFU to conserve their supply.
We will provide any updates as soon as we have them to the on-going supply situation. Thank you for your partnership and understanding as we work through this issue. We would still like to connect with you on call to discuss and review the information as well as our current plans moving forward. We do hope this to be a temporary situation, however, we do want to be transparent with you on what is happening.
Let us know a good time to connect with the Concordance team and we can move forward from there.
Vince Constantine
Associate Director, Channel Management
Front Line Care
M +1.317.691.0586
With any further questions, please reach out to Tricia Wentling.
Kind Regards,
Communication Type:
Product Update
Vendor #10797
-
10/25/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 10-21-2024. Also attached is a usage report for the Low Inventory Adult Institutional Items from the last 6 months.
Please note: There was no usage information for the PSN Deprioritized items or the Adult Inst. Deprioritized items from the last 6 months.
Please see the attachments listed below to reference this notification
- Abbott Institutional Inventory Letter 10_25_24
- Abbott Weekly Inv. Update Item Cross Ref.
- 10-28-2024 Abbott Weekly Inv. Update Item Usage
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
10/25/2024
Title: Health - O - Meter V#10075 - Product UPdate
Details:
Concordance Team,
Please see below for product highlights from Health-O-Meter V# 10075.
Good Morning Tricia,
Happy Friday! Below is a blurb on Antimicrobial Scales to send out to the Sales Team for this upcoming month. Please let us know if you have any questions or if there is anything else we can provide to the team.
Innovating Healthcare with Antimicrobial Scales
Infection control is a top priority in healthcare settings, and Health o meter® Professional Scales is leading the way with their innovative antimicrobial scales. These scales are protected with SterilcoatAM® AM Antimicrobial Powder Coating, which uses silver zinc zeolite technology to inhibit the growth of microbes on powder-coated surfaces.
The Acute Care line of scales were designed to enhance patient and caregiver safety by reducing the risk of infection. Health o meter Professional Scales’ 3105KL-AM & 2900KL-AM feature antimicrobial powder coating on the scale’s painted surfaces as well as an antimicrobial keypad. While several other acute care models also feature the antimicrobial keypad. In addition to antimicrobial powder coatings, equipment can have antimicrobial silver ion additives infused into the plastic parts, such as the handles on the 2210KL-AM neonatal/pediatric scale. By providing a surface that is less likely to contain harmful microorganisms, both the staff and patients are better protected against transmission of a contagion, significantly reducing the risk of a healthcare-associated infection. To learn more about Health o meter Professional’s Antimicrobial scales, visit homscales.com/innovations/antimicrobial-scales
Please feel free to reach out to Brett Lucido, Director of National Accounts, blucido@homscales.com, (724) 799-4233 if you have any questions or would like more information.
Thanks,
Kelly Virzi
Sales and Marketing Specialist
Pelstar LLC/Health o meter® Professional Scales
9500 West 55th Street
McCook, IL 60525-7110 USA
Direct Phone: 708-377-0549
weigheasier®
If you have any further questions, please reach out to Tricia Wentling or Kelly Virzi.
Kind Regards
Communication Type:
Product Update
Vendor #10075
-
10/24/2024
Title: Molnlycke V#10008 - Product Discontinuation
Details:
Molnlycke Warm Up Jackets Immediate Discontinuation Notification
Warm Up Jackets Immediate Discontinuation Item Cross Ref.
Concordance Team,
Molnlycke recently made a decision which impacts their Operating Room Solutions product offering. Molnlycke has informed us that the following Warm Up Jacket SKUs are being discontinued effective immediately. The table below includes the replacement SKUs.
XP Warm Up Jackets SKU Discontinuation & Replacement SKUs
The primary difference between the discontinued SKU and the replacement SKU is the jacket length for the replacement SKU is ~13 inches shorter. Please note, the product price, color and unit of measure are not changing.
Discontinued SKU
Replacement SKU
Size
# of pieces
UPC
Length
(inches)
Width
(inches)
Height
(inches)
Box weight
(pounds)
18200
18001
S
48
07333350716714
210
130
100
2,4
18210
18010
M
48
07333350556068
210
130
115
2,3
18220
18020
L
48
07333350837235
210
130
115
2,4
18230
18030
XL
48
07333350763145
210
130
115
2,5
18240
18040
XXL
48
07333350272029
210
130
130
2,7
18250
18050
XXXL
48
07333350610197
210
130
130
2,8
Attached is the customer notification as well as a Concordance Cross Reference List for the effected items. There was no usage from the last 6 months for the effected items we currently have set up in the system.
With any further questions you may have, please reach out to Tricia Wentling or Mary Reinhart.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10008
-
10/24/2024
Title: Sempermed V#10078 - Product Discontinuation
Details:
SemperGram_Product Update_ORNF SemperForce Orange Retirement
Concordance Team,
Please see the attached for more information on discontinued items from Sempermed V#10078. The effected items are nonstock and listed below:
MFG# CHS# ORNF101
393738 ORNF102
393739 ORNF103
393740 ORNF104
393907 ORNF105
393908 Please note there was no usage from the last 6 months for the affected items.
With any further questions you may have, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10078
-
10/23/2024
Title: Cardinal V#10801 - Greenwood Facility Update
Details:
Concordance Team,
Please see the update below from Cardinal on their Greenwood Facility.
Hello,
Our recovery plan target of 10/21 is extending through this week due to light outbound activity over the weekend. Our Greenwood team is committed to recovering and continue to work overtime plus weekend shifts. We have made significant progress to eliminating the backlog. We project an updated get to health plan starting the week of October 28th.
Thank you for your partnership. We are dedicated to fulfilling orders and getting the right product in the right place at the right time.
Cardinal Health Channel Partners Group
With any further questions regarding Cardinal and this update, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Updated Supplier Information
Vendor #10801
-
10/23/2024
Title: J&J V#10196 - Item Inactivation & Temp. Unavailable Notice
Details:
10-23-2024 Temp. Unavailable Items Usage
Concordance Team,
CHS#146456 MFG#U233H has been placed into item inactivation. We have no QOH to utilize so item should inactivate in the overnight process tonight.
Please also note 3 GUT Sutures are on temporarily unavailable status until Q1 2025. The Buyer (Anne) has placed notes on the items about being unavailable & the ETA.
CHS#515015 mfg#1915G
CHS#645531 mfg#K895H
CHS#138610 mfg#U202H
If you have any further questions, please reach out to Tricia Wentling.
Kind Regards
Communication Type:
Temp Unavailable
Vendor #10196
-
10/23/2024
Title: SC Johnson V#12219 - SPIFF
Details:
Happy-Hands-2024-SC Johnson Professional
Concordance Team,
Please see below from SC Johnson:
Since we have Skincare dispensers and soap on MMCAPs and we are also running a spiff contest on Skincare dispensers -, thought this might be something for your Reps to share with any Schools they call on.
No real commitment but we have had a lot of success with this program and could be a way for schools to buy Skincare from Concordance.
With any further questions, please reach out to Tricia Wentling.
Kind Regards,
Communication Type:
Rep Spiff
Vendor #12219
-
10/22/2024
Title: GOJO V#10354
Details:
GOJO UPDATE - GOJO Heavy Duty Products Supply Update - CONCORDANCE HEALTHCARE SOLUTIONS
Concordance Team,
GOJO Industries sent the following message: "We recognize there has been an emerging, intermittent supply disruption on SKUs within our Heavy Duty portfolio. This is the result of multiple manufacturing-related issues across products like GOJO® Fast Towels, GOJO® PRO™ refills, and GOJO® Hand Cleaner. We have taken action to address these issues and are working to rebuild inventory and support improved service levels. The upcoming production schedules will start improving service levels over the next few weeks, and will continue to improve through November. Please review the attached letter for additional detail."
Of the 32 items on their list, I found nine (9) that crossed to a Concordance item number. However, we have had no sales activity on them during the past six months.
Best regards,
Tricia
Communication Type:
Product Inventory Update
Vendor #10354
-
10/22/2024
Title: Drive Devilbiss V#11048 - Sales Roster Update
Details:
Concordance Team,
Please see the Sales Roster Update from Drive Devilbiss V#11048.
2024 Clinical Care Team Roster
First Name
Last Name
Title
Territory
Phone #
Email
Aaron
Sivley
RSM - Clinical Care
AL, AR, LA, MS, OK
205-440-8912
Eileen
Mack
Corporate RSM - Clinical Care
CO, KS, MO, NM
516-296-0766
Fernando
DeCordova
RSM - Clinical Care
IA, ID, MT, ND, NE, SD, UT, WY
516-757-5007
Jerry
Lyda
Corporate RSM - Clinical Care
IL, MN, WI
516-427-6280
Justin
Bono
RSM - Clinical Care
FL, GA, NJ, NY, PR, SC
818-425-5737
Matt
Herbert
RSM - Clinical Care
CT, DC, DE, MA, MD, ME, NC, NH, PA, RI, VA, VT, WV
516-306-3881
Rachel
Sorensen
RSM - Clinical Care
IN, KY, MI, OH, TN
260-210-4906
Ron
Biles
RSM - Clinical Care
TX
516-563-3790
Sophia
Mateo
RSM - Clinical Care
AK, AZ, CA, HI, ID, NV, OR, WA
949-338-1646
Communication Type:
Sales Roster
Vendor #11048
-
10/22/2024
Title: Coloplast V#10581 - New Item Launch
Details:
Concordance - DAOH12537 - Luja Female Launch Notice - 12.1.24
LowRes-CPCC_Luja_Female_3D Feature and Descriptive images_01
PDAC Approval letter Luja Female- FINAL
PM-32263 Luja Female HCP fact sheet_F
Concordance Team,
Coloplast will be launching four new female catheters from their Luja line effective 12/1/2024. Attached is the information to get the item set-up and ready to sell to our mutual customers.
With any further questions you may have, please reach out to Tricia Wentling.
Kind RegardsCommunication Type:
New Product Launch
Vendor #10581
-
10/22/2024
Title: Abbott V#10575 - Product Disruption
Details:
Glucerna Retail Items Coming Soon Oct 21
Concordance Team,
Please see below and attached on product disruption for Abbott V#10575. First column, ABBOTT#, is item that will be out of stock with no ETA. 3rd Column, Abbott Alternative, is there suggested sub during this stock out. PLEASE REFER TO LETTER FOR DIFFERENCES.
Dear Distributor Partner –
Abbott is committed to providing patients and customers with the essential health care products, services and support they need. The availability of our products is fundamental to meeting that commitment and is a top priority. We are currently forecasting a period of out-of-stock for our Glucerna® Therapeutic Nutrition Shake 8-fl. oz. Abbott Recloseable Container (ARC) products. We offer our sincere apologies for challenges you may experience while sourcing our products.
To better support our valuable Institutional customers, we are increasing production of certain retail SKUs with comparable formulations for institutional usage during this period of low supply. The following product will be made available for our institutions to add to their formularies and for providers to service their patients as Oral Nutritional Supplement options:
-
- Glucerna® Original Shake 8-fl. oz. Recloseable Plastic Bottle (RPB)
These items are being added to your acquisition cost contract pricing as set forth in the table below:
Item #
Description
Units Per Case
Cartons Per Case
Units Per Carton
UPC Case
GTIN Case
Case NDC Format Code(1)
1cs Price Case
125cs Price Case (2)
57804
GLUCERNA SHAKE CREAMY CHOCOLATE DELIGHT 6PK/8 FL OZ BOTTLES
24
4
6
070074578040
00070074578040
70074057804
$55.40
$45.40
57807
GLUCERNA SHAKE STRAWBERRIES AND CREAM 6PK/8 FL OZ BOTTLES
24
4
6
070074578071
00070074578071
70074057807
$55.40
$45.40
57801
GLUCERNA SHAKE VANILLA 6PK/8 FL OZ BOTTLES
24
4
6
070074578019
00070074578019
70074057801
$55.40
$45.40
(1)NDC-format codes: NDC Format codes are product codes adjusted according to standard industry practice to meet the format requirements of pharmacy and health insurance computer systems. Abbott Nutrition does not represent these products to be drugs, nor their codes to be NDC codes. If used for third-party billing purposes, each healthcare provider or contracted vendor is ultimately responsible for the accuracy and appropriateness of codes billed for individual patients and services.
(2) 125 Cs Price / Case: All Abbott products have bracketed pricing based on the quantities ordered which is the price that is reflected on distributor's invoices from Abbott. For Contract Pricing (TN) the 125-case contract price is your Cost-Basis. The Cost-Basis price brackets are the cells outlined in red.
The attached Customer letter is being distributed to our mutual end-user customers beginning this week with direction for them to work directly with their distributor representative on product availability for these items.
We are currently adding Abbott subs but we also have subs listed from Nestle & Kate Farms.
Inventory Control is placing a pause on all affected items.

Please reach out to Mary Reinhart with any further questions you may have.
Kind Regards,
Communication Type:
Product Inventory Update
Vendor #10575
-
-
10/21/2024
Title: Medtronic/Covidien V#10255 - Endo Peanut Discontinuation Notice
Details:
Medtronic Endo Peanut Discontinuation Customer Letter - Final WF 14793012 10.16.24 (002)
Vendor Item List Sales History (9)
Concordance Team,
Please see below and attached on the discontinuance of CHS#301203. Mary has ran usage, but since it has been on BO for so long, it is probably not the most accurate. There are BO's and open PO's across multiple DC's.
Dear Distributor Partners,
The Medtronic Endo Peanut™ instrument (product #173019) was discontinued on 10/16/2024. Please update the product status in your system accordingly.
Due to manufacturing challenges arising from the discontinuation of essential third-party components, product availability has been severely impacted for most of 2024. We regret to inform you that, as a result, this product was discontinued on 10/16/2024. All open orders will be canceled.
The Endo Peanut™ instrument consists of a cotton swab affixed to a 5 mm shaft. It is designed for endoscopic surgery for swabbing small amounts of fluid, or for blunt dissection of soft tissue(s) and structures, or as an aid in controlling minor intraoperative bleeding.
Attachment:
-
- Customer Notification Letter (Oct 2024)
Please forward this message to the appropriate materials management and product master departments within your organization.
Please contact Customer Service at rs.covidiencustomerservice@medtronic.com or (800) 962-9888 with any order related questions.
Thank you.
Brian Hannan
Director, Distributor Sales Operations
Medtronic
60 Middletown Ave. | North Haven, CT 06473 | U.S.A.
Mobile: 203-451-1971
E-Mail: Brian.H.Hannan@Medtronic.com
If you have any further questions, please reach out to Mary Reinhart or Heather Burkett.
Kind Regards,
Communication Type:
Discontinuation Notice
Vendor #10255
-
-
10/21/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 10-21-2024. Also attached is a usage report for the Low Inventory Adult Institutional Items from the last 6 months.
Please note: There was no usage information for the PSN Deprioritized items or the Adult Inst. Deprioritized items from the last 6 months.
Please see the attachments listed below to reference this notification
- Abbott Institutional Inventory Letter 10_18_24
- Customer File Concordance Cross 10-21-24
- 10-23-2024 Abbott Weekly Inv. Update Item Usage
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
10/21/2024
Title: Nestle V#10183 - ThickenUp Product Discontinuation
Details:
ThickenUp (starch) Disco Patient Letter 1024
10-23-2024 ThickenUp Discontinuation Item Usage
Concordance Team,
The items on the attachment are discontinued:
CHS#113566 MFG#22510000 Discontinued and suggested sub CHS#358220 MFG#12498403
CHS#657916 MFG#22540000 Discontinued and suggested sub CHS#191236 MFG#4390015193
CHS#113566 & CHS#657916 have been placed into item inactivation.
With any further questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10183
-
10/21/2024
Title: BD V#10125
Details:
BDX Cumulative Discontinuation Report and Quarterly Concordance Specific Report and Letter
Please see below links
October BDX Discontinuation Letter
BD Cumulative Discontinuations as of Oct 2024
Concordance 2024-10-14 with Tab 2 for customer specifics
Please reach out to Heather Burkett with any questions.
Notification and Customer Usage will be posted on individual items as stock is depleted at BD and IM discontinues each sku since these are happening at different times.
Concordance team,
Please see attached BDX cumulative discontinuation report and the quarterly concordance specific report and associated letter.
October 21, 2024
Dear Valued Customer,
Across BD, we routinely evaluate our product portfolio to ensure we are continuing to meet the needs of our customers – and patients – around the globe. As a result, we will be making strategic decisions affecting our product portfolio. We believe these decisions can offer you a heightened customer experience by increasing the predictability of supply and demand, as well as the speed at which we can deliver products to you. In addition, we believe this will enable the introduction of new, innovative products.
To support your own planning, we want to give you notice of the following product changes. Below is a list of upcoming discontinuations - please refer to the appendix for the specific list of impacted products purchased by your organization, the discontinuation timeline, and recommended alternate BD products.* You and others in your organization may also receive additional information from BD about these upcoming discontinuations.
Material
Discontinued Date
Echo PS™ Inflation Assembly 6 Pack
05/31/2025
Select BD BBL™ Products
Various GasPak™ Accessories
Select BD Phoenix™ Panels
Various BD Macro-Vue™ Products
BD BBL™ Vacutainer® Anaerobic Specimen Collector
Effective Immediately1
Effective Immediately2
04/30/2025
06/28/2025
06/30/2025
Product from the Peripheral portfolio
10/31/2024
BD Alaris ™ Infusion LVP Set
Various Intravenous Access portfolio products
Various Gravity and Syringe Delivery OEM products
Select Texium™ Accessories
05/31/2025
Various Insyte™ Catheters
Select BD Introsyte™ Introducer
06/30/2025
06/30/2025Select BD Integra™ Retracting Safety Syringe with Needles products
Various BD® Weiss Epidural Needles
Select BD® Quincke Epidural Needles
Select BD® Spinal Introducer Needle
Various Spinal Trays
03/31/2024
12/31/2024
12/31/2024
12/31/2024
12/31/2024
Various products from BD Biosciences Research Reagent portfolio (Research Use Only)
04/30/2025
PureWick™ Female External Catheter SKU PWFX30L (contains natural rubber latex)
05/01/2025
1 BD Life Sciences has an ongoing commitment to develop, produce, and deliver the highest quality products to meet your microbiology needs. We have decided to discontinue select BD BBL™ products to focus on providing consistent supply of the suggested alternatives.
2 Raw materials for the parts and accessories associated with the discontinued BD BBL™ GasPak™ Jars have now been depleted.
BD appreciates and values your business and looks forward to your continued interest in our products. While we recognize this change may impact your organization, we are confident that any potential short-term impact will be offset by our commitment to providing innovative products, solutions, and services.
To best serve you, all inquiries and requests should be directed through your local sales representatives or by contacting BD Customer Service at 1-844-823-5433.
Communication Type:
Discontinuation Notice
Vendor #10125
-
10/18/2024
Title: Bowman V#11104
Details:
FLU PROMO
Please see attached end-user PROMO for your customers from Bowman. Details are in sheet and for you to share with your customers. For any additional needs or questions, please contact Bob Kelly or myself.
This will be posted in our Supplier Relations Communications Update Landing page on Connections, or follow this link CLICK HERE.
Thank you everyone!
Bob Kelly
Senior Strategic Account Manager
Marketlab
860.550.1240
Communication Type:
End User Promo
Vendor #11104
-
10/18/2024
Title: Solventum V#10296 - Littmann Stethoscope 2024 Holiday MAP
Details:
Littmann MAP Policy_eff. September 1, 2024
2024 HOliday MAP 10.30-12.04 Item Cross Ref.
10-22-2024 Littmann Map Policy Item Usage
Concordance Team,
Solventum is pleased to announce "2024 Holiday MAP" by lowering MAP on all Covered Products from October 30 - December 4, 2024*.
Please note the MAP Policy remains in effect during this time, and the only change is the lowered MAP on Covered Products. Please see the Holiday MAP highlighted in the following table:
Material Cat# CHS# Billing Unit 2024 MAP 2024 Holiday MAP October 30 - December 4, 2024 3M™ Littmann® CORE Stethoscope, 8480, Black 8480 332854 Each $329.00 $299.00 3M™ Littmann® CORE Stethoscope, 8570, HP Rainbow 8570 357859 Each $349.00 $319.00 3M™ Littmann® CORE Stethoscope, 8870, HP Copper 8870 #N/A Each $349.00 $319.00 3M™ Littmann® CORE Stethoscope, 8890, Mirror 8890 #N/A Each $349.00 $319.00 3M™ Littmann® CORE Stethoscope, 8483, Black, Eko 8483 #N/A Each $329.00 $299.00 3M™ Littmann® CORE Stethoscope, 8574, HP Rainbow, Eko 8574 #N/A Each $349.00 $319.00 3M™ Littmann® CORE Stethoscope, 8838, HP Copper, Eko 8838 #N/A Each $349.00 $319.00 3M™ Littmann® CORE Stethoscope, 8839, Mirror, Eko 8839 #N/A Each $349.00 $319.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Black Tube, 22 inch, 6151 6151 235533 Each $199.00 $184.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Black Tube, 27 inch, 6152 6152 235537 Each $199.00 $184.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Navy Blue Tube, 27 inch, 6154 6154 235540 Each $199.00 $184.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Hunter Green Tube, 27 inch, 6155 6155 235541 Each $199.00 $184.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Plum Tube, 27 inch, 6156 6156 235542 Each $199.00 $184.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Raspberry Tube, 27 inch, 6158 6158 235544 Each $199.00 $184.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Rose Pink Tube, 27 inch, 6159 6159 242502 Each $199.00 $184.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Burgundy Tube, 27 inch, 6184 6184 #N/A Each $199.00 $184.00 Please be advised that this MAP Policy concerns only certain advertised prices and does not relate to the actual sales prices of any product.
*2024 Holiday MAP will start on October 30, 2024 at 12:01 a.m. (EST) and end on December 4, 2024 at 11:59 p.m. (EST).
MAP Policy Update
Please be informed that Solventum has updated the Minimum Advertised Price Policy, effective September 1, 2024 to reflect the company change from 3M Company to Solventum Corporation and the notification period for changes on MAP of any Covered Product is updated as 10 business days.
A copy of the updated Policy is attached.
With any further questions, please reach out to Tricia Wentling.
Kind Regards,
Communication Type:
End User Promo
Vendor #10296
-
10/18/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_18Oct2024
Nestle Weekly Inv. Update Cross Ref.
10-23-2024 Nestle Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 10-18-2024. The following changes are being made:
- Diabetisource AC in 1000 ml was removed
- Boost Kind Essentials Vanilla was removed
- Isosource 1.5 250 ml and 1000 ml was removed
- Novaource Renal 1000 ml was removed
- Peptamen 1.5 250 ml was removed
- Nutren 1.5 1000ml was removed
- Compleat Pediatric Standard 1.4 250ml was removed
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
10/17/2024
Title: Cardinal V#10801 - Greenwood Facility Update
Details:
Concordance Team,
Cardinal has provided us with an update on how things are looking for their Greenwood facility.
Hello,
We continue to work the mitigation plan and remain on track to meet the 10/21 estimated get to health date. Thank you for your patience and partnership as we’ve navigated labor issues, system challenges, learning curves and tropical storms. We are dedicated to getting caught up and staying current to fulfill our customer needs on a regular basis.
Again, thank you for your understanding during this time.
Cardinal Health Channel Partners Group
With any further questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Updated Supplier Information
Vendor #10801
-
10/17/2024
Title: Cardinal V#10801 - Holiday Closure Update
Details:
Holiday Closure Flyer - November 2024 v2
Concordance Team,
Due to the upcoming holiday (Thanksgiving), our Cardinal Health replenishment center will be closed for Business either on Thursday, November 28th or on Thursday, November 28th and Friday, November 29th.
- For the holiday week of the 25th, please place your Monday and Thursday orders as normally scheduled.
- Please place the standard orders, that you would place on the 27th-29th, by Wednesday, November 20th, to supplement orders that would have been placed on the 27th-29th.
Orders placed after the normal cut-off time will be processed on Monday, December 2nd.
For standing delivery appointments(SDAs), adjustments will be coordinated by your transportation replenishment center leader.
With any further questions you may have, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Holiday Schedule
Vendor #10801
-
10/17/2024
Title: Tidi V#10260 - Product Discontinuation Notice
Details:
TIDI Products Discontinued Products Letter - 981413 & 981610 - Sept 2024
10-17-2024 TIDI Product Discontinuation Item Usage
Concordance Team,
Please see discontinued items CHS#106044 (MFG#981413) and CHS#184599 (MFG#981610).
Reach out to Mary Reinhart if you have any further questions.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10260
-
10/17/2024
Title: Cardinal V#10801 - Monoject Sharps Disposal Containers PGII Product Update
Details:
Monoject Sharps Disposal Containers PGII Customer Letter CPG Version
PG2 Table Concordance Cross Ref.
10-22-2024 Monoject Sharps Disposal Containers PGII Item Usage
Concordance Team,
Cardinal has provided the attached customer letter and item list to inform us of updated PGII certified weights for Monoject PGII containers in the 2, 12, 18, & 30 gallon sizes. The newly certified weights will be updated on the label per Department of Transportation (DOT) regulations and Code of Federal Regulations Title 49, part 178.6.
Please see the attached for further information as well as the Item Usage file for the last 6months for the impacted item codes.
With any further questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Product Changes
Vendor #10801
Communication Type:
Product Update
Vendor #10801
-
10/17/2024
Title: Lynn Medical V#11186 - Saline/Sterile Water Inv. Update
Details:
Concordance Team,
Please see the product inventory update from Lynn Medical below.
Good morning All,
I wanted to pass along some information regarding sterile water. Lynn Medical has access to SteriCare Brand Sterile water bottles. If you were ordering Baxter product that was affected by the plant closure this could work as an alternative. Our product only comes in bottles not bags and the second
difference is that our largest container is 1L several of the Baxter products are 2L and 3L. Please see the options listed below as a potential cross reference and let me know if we can help in anyway.
WE also have saline flushes (10ml) available and have included some information on those as well below.
SteriCare Solutions – Saline + Sterile Water:
Part number
description
case qty
cases/pallet
Selling Price
Stock in Michigan
Stock in nashville
Nurse Assist ETA
Qty Pre ordered
Pre-Sold
Balance
6240
(Irrigation Device & Suctioning Saline 0.9%, Sterile 100mL, Screw Top Bottle, 48/cs (Rx) (100 cs/plt
48
100
$46.63
0
0
0
0
0
0
6250
(Irrigation Device & Suctioning Water Sterile 100mL, Screw Top Bottle, 48/cs (Rx) (100 cs/plt)
48
100
$43.48
41 cases
348 cases
in stock
in stock
in stock
in stock
6260
(Irrigation Device & Suctioning Water Sterile 250mL, Screw Top Bottle, 24/cs (Rx) (70 cs/plt)
24
70
$49.10
19 cases
1136 cases
in stock
in stock
in stock
in stock
6270
(Irrigation Device & Suctioning Saline 0.9%, Sterile 250mL, Screw Top Bottle, 24/cs (Rx) (70 cs/plt)
24
70
$51.18
28 cases
789 cases
in stock
in stock
in stock
in stock
5280
(Irrigation Device & Suctioning Saline 0.9% Sterile 500mL, Screw Top Round Bottle, 18/c)
18
64
$60.10
0(taking preorders)
0(taking pre-orders)
28-Oct
356 cases
281 cases
75
5290
Irrigation Device & Suctioning Water Sterile 500mL, Screw Top Round Bottle, 18/cs
18
64
$55.55
0(taking preorders)
0(taking pre-orders)
28-Oct
272 cases
172 cases
100
6281
(Irrigation Device & Suctioning Saline, 0.9%, Sterile, 1000mL, Screw Top Bottle, 6/cs)
6
100
$40.30
0(taking preorders)
0(taking pre-orders)
21-Oct
324 cases
212 cases
112
6291
(Irrigation Device & Suctioning Water, Sterile, 1000mL, Screw Top Bottle, 6/cs)
6
100
$38.18
0(taking preorders)
0(taking pre-orders)
21-Oct
354 cases
268 cases
86
10ml Prefilled Saline Syringes:
Part number
Description
case qty
selling price
stock arriving 10-21-24
pre-sold
balance
IVF1010TM
Amsino Pre-Filled Normal Saline Flush Syringe 10Ml 0.9% Sodium Chloride Fill In 10Ml Syringe 30/Bx 8 Bx/C
240 syringes
$142.56/case
519 cases
150
369 cases
1210BP
Stericare,Sterile 0.9% Sodium Chloride USP, Prefill 10Ml In 12Ml Syringe, 100/Bx, 4Bx/Cs
400 syringes
$272/case
162 cases
19 cases
143 cases
Thank you,
Ryan Milke
Regional Sales Manager, Lynn MedicalIMPORTANT: The contents of this email and any attachments are confidential. They are intended for the named recipient(s) only. If you have received this email by mistake, please notify the sender immediately and do not disclose the contents to anyone or make copies thereof.
With any further questions, please reach out to Jeff Shook.
Kind Regards
Communication Type:
Product Inventory Update
Vendor #11186
-
10/16/2024
Title: Convatec V#10695 - Eakin Product Recall Update
Details:
Recall Notice - Eakin_APPROVED
Certificate of Destruction - TW-1939942_English
10-17-2024 Eakin Product Recall Item Usage
Concordance Team,
A recall communication pertaining to >> Convatec: Eakin Recall - Cohesive Small – TW-1939942 requires your immediate attention.
CHS#101638 (MFG#839002)
Our affected Purchase orders are below:
Billing Document
Purchase order no.
Old material number
Material
Batch
Delivery quantity
Sales Unit
Ship-To Party
Sold-To Party
Name
3051577194
3246365
839002
1012201
109357C553
17
EA
10349155
10349155
CONCORDANCE NEW JERSEY
3051577218
3246370
839002
1012201
109357C553
1
EA
10502797
10349527
CONCORDANCE TIFFIN
3051577425
3246398
839002
1012201
109357C553
10
EA
10349755
10349755
CONCORDANCE SHELBYVILLE
3051577416
3246405
839002
1012201
109357C553
23
EA
10351378
10349527
CONCORDANCE TIFFIN
3051578590
3250778
839002
1012201
109357C553
3
EA
10352117
10349527
CONCORDANCE TIFFIN
3051578258
3250770
839002
1012201
109357C553
12
EA
10352095
10349527
CONCORDANCE TIFFIN
3051578591
3250784
839002
1012201
109357C553
6
EA
10351478
10349527
CONCORDANCE TIFFIN
3051579249
3254792
839002
1012201
109357C553
11
EA
10354166
10349527
CONCORDANCE TIFFIN
With any further questions you may have, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Recall Notification
Vendor #10695
-
10/15/2024
Title: Baxter V#10797 - IV Set Allocation
Details:
IV Sets Allocation Customer Letter 10.14.24 FINAL
Concordance Team,
Please see the attached IV set allocation memo from Baxter. Below are the newly added allocated items from the list. The additional items that are not listed below, were previously allocated and will remain as such. Also attached is the customer listing.
CHS#1448878
CHS#432948
CHS#124216
CHS#115919
CHS#196363
CHS#155790
CHS#224766
CHS#116172
CHS#116826
CHS#985796
CHS#298716
With any further questions you may have, please reach out to Supplier Relations.
Kind Regards
Communication Type:
Allocation Notification/Update
Vendor #10797
-
10/15/2024
Title: Hollister V#10901 - FormaFlex Notice
Details:
September 2024 UPDATE - Hollister FomaFlex Notice
Hollister FormaFlex Notice Item Cross Ref.
10-17-2024 Hollister FormaFlex Notice Item Usage
Concordance Team,
Please see the attached Notice from Hollister. The items were to be discontinued, but that is not the case. The items will remain active SKU's.
They are still active in our system, so this is just an FYI and file for future reference.
With any further questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Product Update
Vendor #10901
-
10/14/2024
Title: Avanos V#11967 - Product Discontinuation
Details:
10-14-2024 Product Discontinuation Item Usage
Concordance Team,
Item Maintenance has notified Supplier Relations that CHS#129132 (MFG#60311) has been placed into discontinuation.
With any further questions, please reach out to Supplier Relations.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #11967
-
10/14/2024
Title: Cardinal V#10801 - Product Discontinuation
Details:
2024 Distributor Material Updates – ADDENDUM 10.11.2024 NON ACUTE
10-15-2024 Product Discontinuation Item Usage
Concordance Team,
Please see the attached and below from Item Maintenance regarding the discontinued SKUs from Cardinal.
These Items have all been placed in item inactivation:
S#183335 Mfg#8887603085S#183434 Mfg#8887603101
S#281436 Mfg#8887605122
S#280867 Mfg#8887605148
S#283291 Mfg#8887605163
S#284794 Mfg#8887605189
S#289256 Mfg#8887605205
S#289363 Mfg#8887605221
S#290056 Mfg#8887605247
S#136341 Mfg#8887605262
S#248104 Mfg#8887631087
S#262576 Mfg#8887630203
S#263491 Mfg#8887630229
S#277319 Mfg#8887630245
These items have all been placed in item inactivation as discontinued.
Communication Type:
Discontinuation Notice
Vendor #10801
-
10/14/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_11Oct2024
Nestle Weekly Inv. Updage Item Cross Ref.
10-16-2024 Nestle Weekly Inv. Update
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 10-07-2024. The following changes are being made:
- Boost Kids Essentials Vanilla was added
- Compleat Pediatric Standard 1.4 was added
- Compleat Standard 1.4 was added
- Isosource 1.5 1000 ml was removed
- Isosource 1.5 250 ml was added
- Peptamen Jr. PHGG was removed
- Compleat Pediatric Peptide 1.5 was added
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
Communication Type:
Discontinuation Notice
Vendor #10863
-
10/11/2024
Title: Mead Johnson V#10502 - Product Inv. Update
Details:
10-15-2024 Mead Johnson Item Usage
Concordance Team,
Mead Johnson products are expanding into distribution, so I will just provide a list of what is not shipping (out of stock).
As of 10/11/2024 (Subject to Change)
Out of Stock MFG# CHS# Product Est. Next Release 166802 261267 Enfamil® 24 Cal 2oz early October 898401 327282 Nutramigen® 6oz (Avail: 898301 Nutramigen 2oz.) end of year 178301 321322 Enfamil® Human Milk Fortifier Liquid High Protein 5.5oz late October 134601 135499 Enfamil® 5% Glucose Water 2oz late October 134503 379496 Enfamil® Sterile Water 32oz (Avail: 134501 2oz. Sterile Water) late December 426201 317744 Enfamil® DHA & ARA Supplement 2oz mid December 433917 350807 Enfamil® Slow-Flow Soft Nipple mid October 433918 350812 Enfamil® Extra Slow-Flow Nipple mid October 210504 333261 Enfamil® Slow-Flow Orthodontic Nipple mid October 28408 341107 Enfamil® Plastic Bottle with Grad-U-Feed Cap 8oz mid October 30061A 356955 Enfamil NeuroPro Infant/Gentlease 7.2oz. Backpack late October 3006AH 379497 Enfamil NeuroPro Enfacare 7.2oz. Backpack late October With any further questions you may have, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Product Inventory Update
Vendor #10502
-
10/10/2024
Title: ICU Medical V#10642 - Smiths Medical Branding Change Update
Details:
SM Name Change - Distributor Messaging_Labeling 2024_FINAL-EN
Concordance Team,
Please review the attached message regarding the distribution of Smiths Medical products.
Kind Regards
Communication Type:
Updated Supplier Information
Vendor #10642
-
10/10/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 10-11-2024. Also attached is a usage report for the Low Inventory Adult Institutional Items from the last 6 months.
Please note: There was no usage information for the PSN Deprioritized items or the Adult Inst. Deprioritized items from the last 6 months.
Please see the attachments listed below to reference this notification
- Abbott Institutional Inventory Letter 10_10_24
- Customer File 10-10-24 Concordance Cross Ref.
- 10-11-2024 Weekly Nutrition Update Item Usage
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
10/10/2024
Title: Steris V#10863 - Product Discontinuation
Details:
Concordance Team,
The below items have been discontinued. Please see suggested replacements.
Discontinued SKU
Description
Suggested SKU
New SKU Description
UOM
Qty/UOM
2024 Acquisition Cost
2025 Acquisition Cost
T0106
Identification Roll Tape Purple 1/8” x 250”
T11006
Identification Sheet Tape Purple 1/8”
ST (SHEET)
1 Sheet
17.06
17.06
T0206
Identification Roll Tape Purple 1/4” x 250”
T12006
Identification Sheet Tape Purple 1/4”
ST (SHEET)
1 Sheet
17.06
17.06
T0306
Identification Roll Tape Purple 3/8” x 250”
T12006
Identification Sheet Tape Purple 1/4”
ST (SHEET)
1 Sheet
17.06
17.06
T0108
Identification Roll Tape Brown 1/8” x 250”
T11008
Identification Sheet Tape Brown 1/8”
ST (SHEET)
1 Sheet
17.06
17.06
T0208
Identification Roll Tape Brown 1/4” x 250”
T12008
Identification Sheet Tape Brown 1/4”
ST (SHEET)
1 Sheet
17.06
17.06
T0308
Identification Roll Tape Brown 3/8” x 250”
T12008
Identification Sheet Tape Brown 1/4”
ST (SHEET)
1 Sheet
17.06
17.06
There was no usage on the effected items in the last 6 months.
If you have any further questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10863
-
10/9/2024
Title: Rockwell V#10934 - Product Discontinuation Notice
Details:
Centrisol and Renasol Concentrate Formulations Catalog
Rockwell Medical Concentrates Product Catalog
Rockwell Medical Product Conversions for PHASE 2 Customer - FINAL
Rockwell_Medical_Product_Conversions 9.30.24
10-09-2024 Product Discontinuation Item Usage
Concordance Team,
Please see the product discontinuation notice below and the associated attachments.
Rockwell Medical will supply you with the following recommended substitute concentrates from our RenalPure® and SteriLyte® product lines:
-
CENTRISOL and RENASOL liquid acid gallons will be substituted with RenalPure liquid acid gallons.
-
CENTRISOL and RENASOL liquid bicarbonate gallons will be substituted with Sterilyte liquid bicarbonate gallons.
Product Substitute Recommendations: Enclosed you will find a comprehensive CENTRISOL and RENASOL product portfolio list along with our recommended RenalPure and SteriLyte product substitutions and product formula details. The products listed in the conversion tables are suggested substitutes only. Please exercise caution, refer to a physician or clinician to confirm appropriate use before administering to a patient, and refer to machine manufacturer for assistance with calibration changes.
-
Rockwell Medical CENTRISOL and RENASOL Product Conversions (Excel)
-
Rockwell Medical CENTRISOL and RENASOL Product Conversions (PDF)
Pricing: Please note that there will be no change to your product pricing at this time. (i.e. you should expect to pay the same price for a RenalPure liquid acid case as a CENTRISOL liquid acid case or RENASOL liquid acid case).
Ordering: Orders should be emailed to our Rockwell Medical Customer Care team at custserv@rockwellmed.com.
Additional information will be provided in the coming weeks. Please contact sales@rockwellmed.com with any questions you have about this transition and refer to RockwellMedical's Product Catalog for additional information on RenalPure and SteriLyte or any other products in our portfolio. We look forward to continuing our business with you.
Sincerely,
Tim Chole
Chief Commercial Officer
Rockwell Medical, Inc.
With any further questions, please reach out to Supplier Relations.
Regards
Communication Type:
Discontinuation Notice
Vendor #10934
-
-
10/9/2024
Title: B. Braun V#10508 - Hurricane Milton Update
Details:
Concordance Team,
Please see the statement from B.Braun below.

With any further questions, please reach out to Supplier Relations.
Kind Regards
Communication Type:
Weather Impact Update
Vendor #10508
-
10/9/2024
Title: Nestle V# 10183 - Product Communication Update
Details: COB Relaunch Side-by-side FINAL (1)
Distributor Price List Effective 12.1.24
Nestle Health Science Customer Communication Letter 10.01.2024 (1)
Oct 2024 Letter-COB 09.19.2024 Spec Sheet (1)
Oct 2024 Letter-Thickenup Disco and COB Cross Reference as of 09.05.2024 (1)
10-09-2024 Nestle Product Communication Item Usage
Concordance Team,
Attached is the Nestle Communication for a multitude of updates regarding new formulations and packaging changes that will replace their COMPLEAT Organic Blends & COMPLEAT Pediatric Organic Blends formulations; Extensive HA Hypoallergenic Infant Formula Packaging Updates; Arginaid Drink Mix Label Update & ThickenUp (Starch-Based) Discontinuation. Please note there was no item usage found for the Extensive HA Hypoallergenic Infant Formula Packaging Updates SKUs from the last 6 months.
Please reach out to Mary Reinhart with any further questions you may have.
Kind Regards
Communication Type:
Product Update
Vendor #10183
-
10/9/2024
Title: Cooper Surgical V#10058 - Product Discontinuation Notice
Details:
10-10-2024 Product Discontinuation Item Usage
Concordance Team,
Please see the attached for further information on discontinued items from Cooper Surgical. Item Maintenance has discontinued the following items and they have been placed in item inactivation.
S#742933 MFG#MXKPCONDS03 disc - suggested sub S#278319 Mfg#MXPCONDS03
S#742936 MFG#MXKPCONDS04 disc - suggested sub Mfg#MXPCONDS04 - replacement not set up due to lack of sales
S#742939 MFG#MXKPCONDS05 disc - suggested sub Mfg#MXPCONDS05 - replacement not set up due to lack of sales
S#245657 MFG#MXKPEH02 disc - suggested sub Mfg#MXPEH02 - replacement not set up due to lack of sales
S#245659 MFG#MXKPEH03 disc - suggested sub Mfg#MXPEH03 - replacement not set up due to lack of sales
S#245660 MFG#MXKPEH04 disc - suggested sub Mfg#MXPEH04 - replacement not set up due to lack of sales
S#245661 MFG#MXKPEH05 disc - suggested sub Mfg#MXPEH05 - replacement not set up due to lack of sales
S#280593 MFG#MXKPER04 disc - suggested sub Mfg#MXPER04 - replacement not set up due to lack of sales
S#286205 MFG#MXKPGE2-1/4 disc - suggested sub S#386442Mfg#MXPGE2-1/4
S#286203 MFG#MXKPGE2-3/4 disc-suggested sub S#405632 Mfg#MXPGE2-3/4
S#286201 MFG#MXKPGE3- disc - suggested sub Mfg#MXPGE3-- replacement not set up due to lack of sales
S#342805 MFG#MXKPGS01 disc - suggested sub MFG# MXPGS01 - replacement not set up due to lack of sales
S#353581 MFG#MXKPGS02 disc -suggested sub MFG#MXPGS02 - replacement not set up due to lack of sales
S#252956 MFG#MXKPINFM disc - suggested sub MFG#MXPINFM - replacement not set up due to lack of sales
S#333132 MFG#MXKPINFS disc - suggested sub MFG#MXPINFS - replacement not set up due to lack of sales
S#742942 MFG#MXKPRS03 disc- suggested sub S#316231 Mfg#MXPRS03
S#742945 MFGEMXKPRS04 disc - suggested sub S#785374 Mfg#MXPRS04
S#785359 MFG#MXKPRSK04 disc - suggested sub S#405630 Mfg#MXPRSK04
S#785362 MFG#MXKPRSK05 disc - suggested sub MFG#MXPRSK05
S#785365 MFG#MXKPRSK06 disc - suggested sub MFG#MXPRSK06
S#785365 MFG#MXKPRSK07 disc - suggested sub MFGEMXPRSK07
For any further questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10058
-
10/9/2024
Title: Cardinal V#10801 - Product Update
Details:
Sharps Safety Phase 2 Customer Letter_October 2024
Cardinal Needles & Syringes Update Item Cross Ref.
10-11-2024 Cardinal Needles & Syringes Update Item Usage
Concordance Team,
The attached letter outlines Phase 2 U.S. manufacturing updates related to our Monoject™ Needles & Syringes. As we continue to ramp up U.S. production of our plastic syringes, we commit to providing regular updates.
Please reach out to Supplier Relations with any further questions you may have.
Kind Regards
Communication Type:
Product Inventory Update
Vendor #10801
Communication Type:
Product Update
Vendor #10801
-
10/9/2024
Title: Healthmark V#11133 - Backorder Notification
Details:
SW-010_Update_Letter_Distributor
Concordance Team,
Please see the attached for further information on the indefinite back order for 10mL Water Leuer Slip Tip Pre-Filled Syringe from Healthmark.
With any further questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Backorder Notification/Update/Reports
Vendor #11133
-
10/9/2024
Title: Nestle V#10183 - Product Discontinuation Notice
Details:
ThickenUp (starch) Disco Patient Letter 1024
Product Discontinuation Item Cross Ref.
10-11-2024 Product Discontinuation Item Usage
Concordance Team,
Please see the follow up information from a previous communication on a product discontinuation from Nestle.
With any further questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10183
-
10/8/2024
Title: Cardinal V#10801 - Greenwood Update
Details:
Concordance Team,
Please see the following regarding Cardinal's Greenwood facility:

With any further questions you may have, please reach out to Supplier Relations.
Kind Regards
Communication Type:
Weather Impact Update
Vendor #10801
-
10/7/2024
Title: Hollister V#10901
Details:
AnchorFast ODT Prdouct Change Notification_Sept 2024
Concordance Team,
Please see the below message and attachment from Hollister:
Dear Valued Customer,
Please see the attached communication regarding updates to Hollister’s AnchorFast™ Oral Endotracheal Tube. The attached document provides relevant details on this change and it’s timing.
As always, please reach out to your Hollister Account Manager or Customer Service at 1.800.323.4060 if you have any questions.
Thank you.
Communication Type:
Product Update
Vendor #10901
-
10/5/2024
Title: Nestle V#10183 - Weekly Nutrition Update
Details:
Abbott Institutional Inventory Letter 10_3_24
Nestle Weekly Nutrition Update Item Cross
10-08-2024 Nestle Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 09-30-2024. The following changes are being made:
- Boost VHC was added
- Isosource 1.5 1500 ml was added
- Peptamen PreBio 1000 ml was added
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
10/4/2024
Title: BD V#10125
Details:
Discontinuation Notice from BD V#10125
Please see attached link for official BD notification, BD Sodium Flouride Tube Discontinuation - Concordance
S#210923 Mfg#368587 is inactive in our system as discontinued.
Mfg#368033 is not set currently set up in our system.
S#718924 Mfg#367729 discontinued and suggested sub S#133829 Mfg#367925.
S#718924 is in item inactivation.
Communication Type:
Discontinuation Notice
Vendor #10125
Communication Type:
New Product Launch
Vendor #10125
-
10/4/2024
Title: Rockwell V#10934
Details:
Important: Product Label (Color) Changes Effective January 6, 2025
HEMODIALYSIS CONCENTRATES LABEL COLOR CHANGE INFORMATION
Please see link above for changes. Thanks,
Communication Type:
Product Changes
Vendor #10934
-
10/4/2024
Title: Cardinal V#10801 - Hurricane Update
Details:
Concordance Team,
Please see below on Cardinal Greenwood trucks effected by the hurricane. It looks like they are up and running but we may experience some delays.

With any further questions you may have, please reach out to Mary Reinhart.
Kind RegardsCommunication Type:
Weather Impact Update
Vendor #10801
-
10/3/2024
Title: Owens & Minor V#12264 - Tariff Update
Details:
20240920_Owens Mino - Customer Letter - Tariffs Update
Concordance Team,
Please see the attached from Owens & Minor regarding Tariff Updates.

With any further questions, please reach out to Supplier Relations.
Kind Regards
Communication Type:
Tarriff
Vendor #12264
-
10/3/2024
Title: Abbott V#10575 - Weelkly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 09-30-2024. Also attached is a usage report for the Low Inventory Adult Institutional Items from the last 6 months.
Please note: There was no usage information for the PSN Deprioritized items or the Adult Inst. Deprioritized items from the last 6 months.
Please see the attachments listed below to reference this notification
- Abbott Institutional Inventory Letter 10_3_24
- Customer File 10-3-24 Concordance Cross Ref.
- 10-04-2024 Abbott Weekly Inv. Update Item Usage
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
10/3/2024
Title: J&J V#10196 - Supply Disruption
Details:
FINAL_Biosurgery Distributor Communication_Supply_Disruption_September 2024.pdf
10-07-2024 Supply Disruption Item Usage
Concordance Team,
J&J is experiencing supply disruption to the Specific Surgifoam product codes listed below resulting in shipping delays.
MFG# VAI Item Description Size 1972 531152 Surgifoam Absorable Gelatin Sponge 2cm x 6cm x 7mm (12 - 7) 1973 N/A Surgifoam Absorable Gelatin Sponge 8cm x 6.25cm x 10mm (50) 1974 155466 Surgifoam Absorable Gelatin Sponge 8cm x 12.5cm x 10mm (100) 1975 168267 Surgifoam Absorable Gelatin Sponge 8cm x 12.5cm x 2mm (100c) Please see the attached for further information.
With any further questions, please reach out to Supplier Relations.
Kind Regards
Communication Type:
Backorder Notification/Update/Reports
Vendor #10196
-
10/2/2024
Title: Stryker Instruments V#10912 - Recall Notice
Details:
10.2.24 RECALL_Stryker Instruments_Copy of Affected_Distributor_Locations-Concordance
SafeAir Customer Notification Letter - Final
SafeAir Distributor Notification Letter - Final
Concordance Team,
Stryker sent the following message:
This notification is to inform you Stryker Instruments is voluntarily recalling specific lots listed in the attached notification letter for our SafeAir Smoke Evacuation Pencil.
The following documents are attached:
- Affected_Distributor_Locations.xlsx: Identifies locations to which Stryker may have shipped affected product and associated lots.
- SafeAir Distributor Notification Letter – Final.pdf: Contains instructions specific to Stryker’s distributor and/or kitting partners. Not intended to be forwarded to end customers.
- SafeAir Customer Notification Letter – Final.PDF: Distributors will send this version of the voluntary recall notice to our mutual end-customers.
All SafeAir shipments made after 09/19/2024 do NOT contain affected product and can be distributed to our mutual end-customers.
We understand our distributors/kitters will need to quarantine inventory while it is reviewed to identify affected lots and this could impact our customer’s ability to purchase these SafeAir SKUs. Stryker is prepared to partner with you in support of maintaining supply continuity for our mutual end-customers. We would like to discuss your specific inventory needs and how best to align. Please let us know 2-3 30-min blocks for this week when you and your team can be available to meet to discuss further.
If you have any additional questions, please feel free to reach out to me or directly to Instruments.Recalls@stryker.com.
Communication Type:
Recall Notification
Vendor #10912
-
10/2/2024
Title: BD V#10125 - Port Strike Update
Details:
BD Statement Port Strike 9-20-2024.pdf
Concordance Team,
BD has provided us with the attached statement on Potential East and Gulf Coast Port Strike. Please review for more information.
With any further questions, please reach out to Supplier Relations.
Kind Regards
Communication Type:
Strike Update
Vendor #10125
-
10/2/2024
Title: Owens & Minor V#12264 - Port Strike Update
Details:
Concordance Team,
Please see the Port Strike Updated from Owens & Minor below:

With any further questions you may have, please reach out to Supplier Relations.
Kind Regards
Communication Type:
Strike Update
Vendor #12264
-
10/2/2024
Title: Medical Action V#10045 - Hurricane Update
Details:
Concordance Team,
Please read the following pertaining to Medical Action's Asheville, NC facility.
First Alerts
As of 10/1/24
Hurricane Helene Recovery: An Update on our Asheville, NC Operations
With recovery efforts ongoing throughout the Southeast following Hurricane Helene, we are sharing an additional update with our customers on the status of Owens & Minor’s operations in Asheville, NC.
Our highest priority is on the safety and wellbeing of the over 400 teammates that live and work in Asheville and the surrounding areas. With local communications disrupted, Owens & Minor is still in the process of confirming the safety and whereabouts of many of the teammates that work in our Asheville facility. What we do know is that like so many others, some of our teammates are experiencing devastating personal losses.
While we have confirmed that our Asheville site and the products stored inside were not damaged, Owens & Minor continues to assess the situation as our operations and emergency response teams work to bring local operations back online. From an operations perspective, we are able to provide the following updates:- We are in the process of reestablishing utility services to the facility; however, we have not been given an exact timeline for when power and water service will be restored.
- Our emergency response team is in contact with state and local officials including the Federal Emergency Management Agency (FEMA) to ensure that we receive critical updates about the broader recovery response.
- We have secured accurate inventory positions within our Owens & Minor distribution network.
- We are actively coordinating with our distribution partners to obtain inventory positions for Medical Action products within their distribution network.
- At this time, we do not anticipate immediate service level impacts.
Our thoughts are with our teammates, the frontline humanitarian and emergency responders, and the entire Buncombe County community as they navigate this difficult time. Owens & Minor is committed to providing customers with timely information about our Asheville operations, and we will share additional updates as the recovery continues.With any further questions, please reach out to Supplier Relations.
Kind Regards,
Communication Type:
Weather Impact Update
Vendor #10045
-
10/2/2024
Title: Coloplast V#10581 - Port Strike Update
Details:
Concordance Team,
Port Strike Notification: Coloplast is working to mitigate potential complications due to the port strike. To reduce risk of interruption of product supply, Coloplast Global teams have established plans for air shipping in the event we face low stock or backorders of items, however, our current supply of products is healthy.
With any further questions, please reach out to Mary Reinhart.
Kind Regards,
Communication Type:
Strike Update
Vendor #10581
-
10/2/2024
Title: Alimed V#11112 - Product Discontinuation
Details:
Concordance-AliMed Price File Effective 1-1-2025_12-31-2025
10-02-2024 Product Discontinuation Item Usage
Concordance Team,
Attached please find your new price file for AliMed that will be effective 1/1/2025-12/31/2025.
Effective 12/31/2024, please expire ALL existing pricing for any AliMed items that you may have in your systems. If there are items that Concordance wishes to purchase from AliMed that are not identified in the attached file, please reach out to Pricing@alimed.com for price verification.
Please note that there are 2 separate tabs contained in this price file as described below:
- Active AliMed items with your new pricing to be loaded 1/1/2025
- A list of items that have been discontinued and should be removed from your systems. Applicable substitute items have been added.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #11112
-
10/2/2024
Title: ICU Medical V#10917 - Allocation Update
Details:
IV Solution Supply Update_Distributor Letter _ 10012024
Concordance Team,
The attached items have been added onto allocation today(10-2-24), customer allocations will be added in overnight. A customer listing is attached of those using who will need notified.
Hurricane Helene IV Solutions Key Points:
- ICU Medical’s Austin, TX IV Solutions manufacturing facility was unaffected by Hurricane Helene and is continuing to operate and produce IV Solutions as normal
- We have placed our IV Solutions portfolio on 125% protective allocation based on ICU Medical customer usage from Jan ‘24 – Jun ‘24 to help ensure uninterrupted supply for our contracted and loyal customers, including trading partners
- It is our goal to help ensure that our loyal customers remain unaffected by this tragedy
- We have already taken necessary steps to increase IV Solutions production appropriately in response to market needs and protecting inventory for our customers
- We will continue to provide ongoing customer updates as we continue to collaborate with government agencies
Kind Regards
Communication Type:
Allocation Notification/Update
Vendor #10917
-
10/2/2024
Title: Medical Action V#10045 - Inventory Update
Details:
Good Afternoon All,
Please see below from Owens&Minor on updates from Hurricane Helene.
- Their Asheville, NC operations is without power, but inventory and building is safe.
- They do not expect inventory disruptions at this time.
- Additional details below.
We will keep the team updated as we receive any additional updates.
Thank you,
Communication Type:
Product Inventory Update
Vendor #10045
-
10/2/2024
Title: Baxter - Allocation Update
Details:
Allocation Letter_HH_10.1.2024 FINAL
Customer Communication_Hurricane Helene Impact
IV Solution Supply Update_Distributor Letter _ 10012024
Concordance Team,
Please see the attached communications from Baxter, B.Braun and ICU Medical on current allocations being put into place today. If customers are ordering product that they previously haven’t ordered we need to cancel the orders. Today our goal is to protect committed customers inventory.
- Baxter it is either 10% or 100% on products March – August sales (if you see a % different than 100% that means we are getting 10% allocation)
- B.Braun is 100% for B.Braun committed customers and nothing to anyone else
- ICU Medical is 125% using January – June sales
After the allocation process runs again this evening we will be able to send out customer allocation files.
We are suggesting the CHS reps reach out directly to the appropriate local ICU/B.Braun sales rep to see if they can get allocation for customers. We are trying internally but the voice of the customer is much louder than ours at this time.
Communication Type:
Allocation Notification/Update
-
10/2/2024
Title: B.Braun V#10508 - Allocation Update
Details:
Concordance Team,
The attached items have been added to allocation today, customer allocations will be added in overnight. A customer listing is attached of those using who will need to be notified.
Currently, B Braun has these items set at 100% allocation and those will only go to B. Braun contracted customers. We cannot release our stock to anyone to use as a sub. If they are contracted to B. Braun and using then they should have an allocation loaded.
Thanks,
Communication Type:
Allocation Notification/Update
Vendor #10508
-
10/1/2024
Title: Molnlycke V#10008 - China Tariffs Update
Details:
Concordance Team,
Please see the information below from Molnlycke regarding the China Tariffs.
Dear Valued Distributor,
Molnlycke would like to inform you that, effective September 27, 2024 additional tariffs will be levied on a range of medical products imported from China, following recent U.S. Government policy updates. These tariffs are expected to significantly impact costs for many suppliers.
However, we are pleased to confirm that Mölnlycke’s Biogel® surgical gloves are not sourced from China. All Biogel® gloves are manufactured in our state-of-the-art facility in Malaysia, a recognized center of excellence for glove production and the upcoming tariff changes will not affect the pricing or availability of Biogel® gloves.
At Mölnlycke, we remain committed to supporting our customers with high-quality products and ensuring a reliable, uninterrupted supply. Your trust and partnership are our top priority, and we will continue to navigate any challenges to meet your needs.
Please feel free to contact our Customer Experience team at customer.orders@molnlycke.com, or by phone at 1-800-843-8497 with any questions. Attached is our update notification, which can be distributed among your team members.
Sincerely,
Bob Perry
Director, US Channel Management
With any further questions you may have, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Tarriff
Vendor #10008
-
10/1/2024
Title: Molnlycke V#10008 - Potential Strike Update
Details:
Molnlycke Customer Communication_Potential Strike 9.27.24.pdf
Concordance Team,
Mölnlycke is closely following the ongoing negotiations between International Longshoremen’s Association (ILA) and the United States Maritime Alliance (USMX) and the potential strike action planned for October 1st at the East and Gulf Coast ports of the U.S.. We have a taskforce in place to monitor the situation and are working closely with our logistics partners in order to mitigate any potential disruptions to the supply chain should the strike ensue.
Molnlycke is committed to keeping you informed as the situation evolves. Please see the attached notification for more detailed updates on each of our product portfolios.
If you have any further questions, please reach out to Mary Reinhart.
Kind RegardsCommunication Type:
Strike Update
Vendor #10008
-
10/1/2024
Title: ICU Medical V#10917 - Port Strike Update
Details:
Port_Strike_Customer_Communication_2024_09_Final
Concordance Team,
ICU Medical V#10917 has provided the attached communication regarding the current Port Strike.
With any further questions you may have, please reach out to Supplier Relations.
Kind Regards,
Communication Type:
Strike Update
Vendor #10917
-
10/1/2024
Title: Avanos V#11967 - Port Strike Update
Details:
Concordance Team,
Please find the below statement from Avanos Medical's Global Transportation Team regarding the Potential Impacts and Mitigations from the Port Strike. They will provide additional details as it is made available.
To: SLT; Sales, Operations leaders (please share as appropriate within your teams
All:
As you may be aware, a potential strike by dockworkers at US East and Gulf Coast ports could begin as early as Tuesday, Oct. 1. Given the disruptions that could accompany such a strike, we wanted to share information on what Avanos is doing to mitigate the potential impact on our ability to fulfill customer orders.
What’s the background on this issue?
Dockworkers from Maine to Texas, represented by the International Longshoremen’s Association, are at odds with port authorities on a variety of issues, including wages, working conditions, job security and increasing automation of port operations. A strike would shut down as many as 36 ports, impacting both import and export supply chains.
How would a potential strike impact Avanos and our customers?
Avanos exports products from the US via both air and ocean freight. The vast majority of our shipment volumes support shipping via air freight, so a strike would have little impact on those shipments.
However, we do ship via ocean freight from US distribution centers (DCs) to three international DCs in Australia, Japan and, most significantly, Belgium. A strike would potentially delay stock transfer order shipments to those international DCs.
Can’t we re-route ocean shipments via other ports?
Re-routing is generally not a good option for us. Given a strike, all East Coast ports would have some potential impact, and ports on the US West Coast are already congested.
What is Avanos doing to mitigate this issue?
We are closely monitoring the situation and preparing as best we can, but for now, we must wait to see how these labor negotiations proceed. In the meantime, our Transportation team is working closely with our International Supply Chain team to expediate products by shifting from ocean to air freight, where necessary. We typically have a buffer and healthy safety stocks for products shipped via ocean freight, which would allow us to absorb a small delay (one or two weeks), should the strike occur.
With any further questions you may have, please reach out to Supplier Relations.
Kind RegardsCommunication Type:
Strike Update
Vendor #11967
-
10/1/2024
Title: Baxter - Allocation Update
Details:
CHS Update on Baxter 10.1.2024
Medical Assessment Sept 30 FINAL5pm[2]
Concordance Team,
Please see the information below and attached regarding the Baxter Allocations.
On behalf of Missy Depinet:
Team,
As you may know, Baxter’s North Cove, NC facility has closed, which has impacted product availability for several Concordance warehouses. We’ve sent a customer communication to our sales team, outlining our strategy to manage this situation (refer to the attached "CHS Update on Baxter 10.1.2024").
In addition, here’s a detailed overview of the internal measures we are taking and important items you need to be aware of:
-
- Medical Assessment: Baxter has issued a letter providing guidance on the management and conservation of affected products. Please refer to this document for more information.
- Impacted Customer List: Attached is a list of Concordance customers who purchase items on the 48-hour hold list. These customers are directly impacted and will need careful coordination. Please do not share this list outside of Concordance.
- 48-Hour Hold Codes: We’ve included a list of items currently on hold as part of the 48-hour hold process.
- SUBS 10.01.2024 PM: This document outlines the known substitutions for products we sell that are on the 48-hour hold list. Please work closely with your accounts to ensure they are aware of these subs.
- Substitution Notification: If a customer decides to switch to a substitute product, please notify Amy Jordan and Darrin Werner so we can begin securing additional inventory. While suppliers are currently not placing substitutes on allocation, this situation is likely to change soon, so proactive communication is crucial.
- CHS Days On Hand (DOH): For reference, we’ve included a network-wide DOH report to provide insight into product availability across CHS warehouses. This can be helpful when discussing options with customers.
- Baxter Allocations: Baxter has not yet finalized allocations for the impacted products, but we expect this to be done by the end of the week. Once available, these final allocations will replace the provisional figures in our system.
- Thank you for your cooperation as we navigate this situation.
- Missy Depinet
- VP, Procurement & Inventory
- Concordance Healthcare Solutions
Communication Type:
Allocation Notification/Update
-
-
10/1/2024
Title: O&M Halyard V#12264 - Port Strike Update
Details:
Concordance Team,
Owens & Minor - Halyard V#12264 has notified us of the following regarding the Port Strike:
East & Gulf Coast Port Strike:
As multiple news outlets including the New York Times are reporting, as of today the International Longshoremen's Association (ILA) has initiated a work stoppage halting most container operations at 36 ports along the U.S. Easter seaboard and Gulf Coast.
Although the situation continues to evolve and delays may not be felt immediately, Owens & Minor is highly focused on mitigating the impact of the strike and we are committed to working hand-in-hand alongside our customers to help maintain business continuity. To that end, we have activated our emergency counter response plan and taken several measures to help support our customers should the strike continue:
- Increasing production and availability of our proprietary HALYARD Brand products, which are manufactured in the Americas, to help meet customer demand for key products.
- Coordinating with out supplier network on potential contingency plans to help ensure product availability where possible.
- Shifting inbound shipments that were originally destined for East Coast ports to West Coast ports, when possible.
- Expediting shipments arriving by ocean and redirecting them to the ports of Los Angeles and Long Beach.
- Leaning further on our domestic transportation and railroad partnerships to rapidly deploy and rebalance inventory within the U.S.
More information is to come.
With further questions, please reach out to Supplier Relations
Kind Regards
Communication Type:
Strike Update
Vendor #12264
-
09/29/2024
Title: BD V#10125 - Product Coming off MBO
Details:
10-03-2024 Product Inv. Update Item Usage
Concordance Team,
Please see the email below regarding the BD Urine Kits coming off MBO. I have attached usage for the affected product from the last 6 months.
Good afternoon Concordance team, Below Is a Urine Kit update.
Our BD Vacutainer® complete urine collection kits have cleared backorder and supply outlook is positive to support current business and get us back to healthy inventory levels.
Based the positive outlook it is very exciting to officially share that we are ready to reactivate conversions and take on new business for the following products:
-
- 364956 (CHS#368837)- Urine cup, C&S and UA tubes
- 365957 (CHS#775403)- Urine cup, C&S and UAP tube
Thank you
Please reach out to Heather Burkett with any further questions you may have.
Kind Regards,
Communication Type:
Backorder Notification/Update/Reports
Vendor #10125
-
-
09/27/2024
Title: Convatec V#10695 - Product Recall Notice
Details:
Concordance Team,
Convatec has provided information pertaining to their Product Recall for MFG#839002 (CHS#101638). For further information, please see the attached Recall Notice. Certificate of Destruction, and Item Usage Report for the affected products.
The affected purchase orders are below:
Billing Document
Purchase order no.
Old material number
Material
Batch
Delivery quantity
Sales Unit
Ship-To Party
Sold-To Party
Name
3051577194
3246365
839002
1012201
109357C553
17
EA
10349155
10349155
CONCORDANCE NEW JERSEY
3051577218
3246370
839002
1012201
109357C553
1
EA
10502797
10349527
CONCORDANCE TIFFIN
3051577425
3246398
839002
1012201
109357C553
10
EA
10349755
10349755
CONCORDANCE SHELBYVILLE
3051577416
3246405
839002
1012201
109357C553
23
EA
10351378
10349527
CONCORDANCE TIFFIN
3051578590
3250778
839002
1012201
109357C553
3
EA
10352117
10349527
CONCORDANCE TIFFIN
3051578258
3250770
839002
1012201
109357C553
12
EA
10352095
10349527
CONCORDANCE TIFFIN
3051578591
3250784
839002
1012201
109357C553
6
EA
10351478
10349527
CONCORDANCE TIFFIN
3051579249
3254792
839002
1012201
109357C553
11
EA
10354166
10349527
CONCORDANCE TIFFIN
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Recall Notification
Vendor #10695
-
09/27/2024
Title: Steris V#10863 - Product Discontinuation Notice
Details:
Reliance 333 washer Dedicated Chemistries Discontinuation Letter
Steris Discontinuation Product Cross Ref.
10-01-2024 Steris Discontinuation Item Usage
Concordance Team,
Effective December 31, 2024, STERIS will no longer accept orders for the dedicated chemistries used in the Reliance 333 Washer/Disinfector. The Reliance 333 Washer/Disinfector has not been sold since 2013 and has not been serviceable since 2022.
- RENU-KLENZ™ Neutral Instrument Detergent
- 1777BX – Renu-Klenz (4 x 1 Gal bag-in-box-case) - Item available until 12/31/2024 or until inventory is depleted
- 1777BXEC – Renu-Klenz / Euro (4 x 3.78L bag-in-box-case) - No longer available
- Hinge-Free™ Instrument Lubricant
- 1031BX – Hinge-Free (4 X 1 Gal Bag-In-Box-Case) - No longer available
- 1031BXEC – Hinge-Free/Euro 4X3.78L B-I-B (Bag-In-Box) - No longer available
- Klenzyme™ Enzymatic Presoak and Cleaner
- 1673BX – Klenzyme Enzymatic Presoak Cleaner (4 x 1 gallon per case) - No longer available
- 1673BXEC – Klenzyme / Euro (4 x 3.78L bag-in-box-case) - No longer available
There are no alternatives listed.
With any further questions, please reach out to Mary Reinhart.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10863
-
09/27/2024
Title: Mead Johnson V#10502 - OOS Notice
Details:
Concordance Team,
Mead Johnson product is expanding into distribution, so I will just provide a list of what is not shipping (out of stock). Most on the list should be back next month. This list is subject to change.
CHS# MFG# Product Est. Next Release 227309 156401 Enfamil® Premature 20 Cal 2oz early October 227313 156601 Enfamil® Premature High Protein 24 Cal 2oz early October 261267 166802 Enfamil® 24 Cal 2oz early October 327282 898401 Nutramigen® 6oz (Avail: 898301 Nutramigen 2oz.) end of year 101967 20102 Enfamil® A.R. Powder 12.9oz 322745 178701 Puramino Junior Unflavored Powder 14.1oz late September 321322 178301 Enfamil® Human Milk Fortifier Liquid High Protein 5.5oz late October 135499 134601 Enfamil® 5% Glucose Water 2oz late October 379496 134503 Enfamil® Sterile Water 32oz end of year 350812 433918 Enfamil® Extra Slow-Flow Nipple mid October 350807 433917 Enfamil® Slow-Flow Soft Nipple early Oct. + late Oct 333261 210504 Enfamil® Slow-Flow Orthodontic Nipple early October 317744 426201 Enfamil® DHA & ARA Supplement 2oz December 356955 30061A Enfamil NeuroPro Infant/Gentlease 7.2oz. Backpack late October 379497 3006AH Enfamil NeuroPro Enfacare 7.2oz. Backpack late October Communication Type:
Product Inventory Update
Vendor #10502
-
09/27/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_27Sep2024
Nestle Weekly Inv. Update Item Cross Ref.
10-02-2024 Nestle Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 09-16-2024. The following changes are being made:
- Boost VHC Chocolate
- Isosource 1.5 250mL
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
09/26/2024
Title: Hollister V#10901 - Product Discontinuation Notice
Details:
HOL US Restore Hydrocolloid Discontinuation Letter
Product Discontinuation Item Cross
09-26-2024 Product Discontinuation Item Usage
Concordance Team,
Please see the attached important update regarding the Hollister Wound Care product portfolio. Also attached is usage regarding the affected items for the last 6 months.
Kind Regards,
Communication Type:
Discontinuation Notice
Vendor #10901
-
09/26/2024
Title: Parker Labs V#10066 - Product Catalog
Details:
Parker Labs CAT - DO - 000 REV WEB 13 3-7-24_LOCKED
Concordance Team,
Please see Parker Labs V#10066 latest product catalogue for your review.
Kind Regards
Communication Type:
Product Catalog
Vendor #10066
-
09/26/2024
Title: First Quality Products V#10968 - NuFit Brief Discontinuation
Details:
09-27-2024 NuFit Product Discontinuation Item Usage

With the introduction of our NEW reimbursement-friendly 20-count packs in November 2024 and to maximize efficiencies, First Quality® will discontinue Prevail® Nu-Fit® Maximum Absorbency Adult Brief standard case counts in March 2025. This includes the following item
numbers: NU-012/1, NU-013/1, and NU-014/1
Prevail® Nu-Fit® Briefs will still be available in our reimbursement-friendly counts and as well as our NEW size 2X-Large (CRB-017). Please reference page 2 for Case and Pallet information.
Per each pricing will stay the same. For any additional questions on pricing, including information on uniform into stock pricing or adjustments to rebate contracts, please contact your First Quality® sales ambassador.



If you have any further questions, please reach out to Heather Burkett for further information.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10968
-
09/25/2024
Title: Guerbet V#12051 - Urological Imaging Product Update
Details:
Project Urban 20240722 - Press Release
09-27-2024 Project Urban Item Usage
Concordance Team,
Guerbet has put out information regarding the sale of their Urological Imaging Systems Business Assets to Del Medical. Please see the attached Press Release from Guerbet for further information. Also attached is a usage report to show the impacted customers and their account managers.
Impacted Product SKUs:
MFG# CHS# Description 335563 304501 URO DRAIN BAGS-STERILE (CTN-10); 10EA/CA 335825 846329 DISP DRAIN BAG (10) NONSTERILE; 10EA/CA 337125 154010 UNIVERSAL FOOTSWITCH CVR DISP; 25EA/CA 337200 #N/A Y-TUBES, CARTON OF 10; 10EA/CA 337205 #N/A URO DRAIN EXT TUBING (CA/10); 10EA/CA 337210 #N/A FLUIDRAIN LSS 10; 10EA/CA 337220 #N/A FLUIDRAIN LSS 20; 10EA/CA With any further questions, please reach out Mary Reinhart.
Kind Regards,
Communication Type:
Updated Supplier Information
Vendor #10251
-
09/24/2024
Title: Medtronic V#10255 - Product Unavailability Update
Details:
Medtronic MultiFire Premium Skin Stapler and Reload Backorder Letter_9.20.24
09-26-2024 Medtronic MBO Item Usage
Concordance Team,
Medtronic regrets to inform us that the MultiFire Premium™ Single Use Skin Staplers (MFG# 059037/CHS#281246) and Reloads (MFG# 059038/CHS#300859) will be unavailable through June 2025 due to a supply constraint.
The recommended substitution is the Appose™ ULC Skin Stapler with 35 Wide Staples (item # 8886803712/CHS#484386).
Thank you
Communication Type:
Backorder Notification/Update/Reports
Vendor #10255
Communication Type:
Temp Unavailable
Vendor #10255
-
09/20/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_20Sep2024
09-24-2024 Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 09-16-2024. The following changes are being made:
- Diabetisource AC 1500 ml was added
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
09/20/2024
Title: Graham Medical V#11028 (Slid to V#10030) - September Marketing
Details:
Concordance Team,
This month’s highlight from Supplier partner Graham Medical!! Reach out to your local rep or feel free to contact Greg Lange directly with any questions or assistance needed.
Thank you!!
Communication Type:
Product Flyer
Vendor #11028 (Slid to V#10030)
-
09/19/2024
Title: Molnlycke V#10008 - New Product Notice
Details:
Mepilex Border Post - Op Additional Sizes Distributor Notifications 9.18.24
Concordance Team,
Molnlycke is pleased to announce that two additional sizes of Mepilex Border Post-Op are being introduced into their Mepilex Border Post-Op portfolio.
Please note that the two new sizes Mepilix Border Post - Op SKUs are available immediately. Details of the new smaller dressings are as follows:
MFG# Description Dimensions 496100 Mepilex Border Post Op 2.5x3in (6x8cm) 496200 Mepilex Border Post Op 3.5x4in (9x10cm) Details about this new additions can be found in the official Distributor Notification letter attached. These codes have been added to all Molnlycke-awarded GPO Wound Care contracts.
Please contact Mary Reinhart for further information or questions you may have.
Kind Regards,
Communication Type:
New Product Launch
Vendor #10008
-
09/19/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 09-19-2024. Also attached is a usage report for the Low Inventory Adult Institutional Items from the last 6 months.
Please note: There was no usage information for the PSN Deprioritized items or the Adult Inst. Deprioritized items from the last 6 months.
Please see the attachments listed below to reference this notification
- Abbott Institutional Inventory Letter 9_19_24
- Customer File - Concordance Cross Ref.
- 09-24-2024 Weekly Inv. Update Item Usage
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
09/18/2024
Title: Cardinal V#10801 - Ultrasound Gel Product Discontinuation Notice
Details:
Ultrasound Gels Discontinuation_Customer Letter 8.16.24
Ultrasound Gel Discontinuation Item Cross Ref.
09-18-2024 Ultrasound Gel Discontinuation Item Usage
Concordance Team,
Cardinal has informed us of a unexpected, long-term supply disruption on Cardinal Health brand Ultrasound Gels due to their supplier's decision to discontinue production.
These products will continue to be offered to customers until inventory is depleted. Please see the attached Cross Reference List and Customer Letter for impacted Cardinal Health brand material numbers and CHS#s, recommended alternatives, and estimated inventory depletion dates.
While Cardinal will no longer have a Cardinal Health branded Ultrasound Gel, they are committed to providing customers will high-quality products for women and Baby care.
With any further questions you may have, please reach out to Bill Black.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10801
-
09/17/2024
Title: BD V#10128 - End User Promo
Details:
3417 BD Veritor Triplex Half page
End User Promos Item Cross Ref.
9-17-2024 BD End User Promo Item Usage
Concordance Team,
BD is kicking off their respiratory season July 1, 2024. Here are a few of the sell sheets to start off, but there are more to come by the end of the month.
The 2 new SKUs this year are:
MFG#256132 Pediatric Value Pack - (2Triplex kits and 2 RSV Kits) at discounted price.
MFG#256129 Urgent Care Value Pack - (2Triplex Kits, 1 Flu Kit and 2 Strep Kits) at a discounted price
Please see the cross reference list and Item Usage file for each promo to see the affected customers.
With any further questions you may have, please reach out to Bill Black.
Kind Regards
Communication Type:
End User Promo
Vendor #10128
-
09/16/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_13Sep2024
Nestle Weekly Inv. Update Item Cross Ref.
09-16-2024 Nestle Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 09-09-2024. The following changes are being made:
- Boost Plus Vanilla was added
- Boost VHC Chocolate was added
- Compleat Pediatric Standard 1.4 was added
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
09/16/2024
Title: Solventum V#10296 - SoluPrep S Cross Reference to BD
Details:
Surgical Preps Customer Letter_Sept 13 2024
09-16-2024 SoluPrep S Item Usage
Concordance Team,
BD has provided information that they have stock available for SoluPrep S Skin prep to aid in industry shortages. Please read below from Solventum and the attached for further information and product discount information.
We have received quite a few inquiries from customers the past few days about a shortage in the market of Surgical Patient Skin Preps.
We would like to let our distributors know that we have a good supply available of SoluPrep S Sterile Antiseptic Solution with the same active ingredients, intended uses, and application process as the leading surgical prep, and it is readily available to order with no quantity limits at this time.
We can offer a 20% discount to help make this available product to our mutual customers.
- Below are the quantities available and expiration dates.
- Terms: Available first come, first served. Product is non-returnable (drug products are not returnable). 20% discount available to authorized Solventum distributors, while supplies last, on purchase orders placed and received between 9/16/2024 – 12/31/2024.
- A customer letter is attached with the cross-reference and additional product information for new users of SoluPrep S.
- The product application process is the same as the leading surgical prep, with a slightly different applicator activation technique. Our website contains the complete Drug Facts detail and an Application Video, to provide on-demand training for users. www.3M.com/solupreps
- Clinical support is available from our field sales team, surgical infection prevention specialists, and clinical specialists.
Available For Supplemental Purchase
Product
Code
Available in Eaches
Available in Cases
Expiration Dates
List Price
Soluprep S
67130
79,800
3,990
2025-Mar
172.20
10,600
530
2025-May
172.20
9,600
480
2025-Jun
172.20
Soluprep S
67134
58,500
1,170
2025-Mar
228.00
Soluprep S
67133
20,100
402
2025-May
228.00
33,900
678
2025-Jun
228.00
Total
212,500
7,250
Surgical Patient Skin Preps Cross-Reference
Surgical Prep
Solventum Cross-Reference
Catalog Code
Product Description
Catalog Code
Product Description
930700
Skin Prep Solution ChloraPrep™ Clear 10.5 mL Applicator 2% CHG / 70% IPA (Chlorhexidine Gluconate / Isopropyl Alcohol)
67134
3M™ SoluPrep™ S Sterile Antiseptic Solution, 2% CHG / 70% IPA (Clorhexidine Gluconate / Isopropyl Alcohol) Surgical Prep, 67134, Clear, 10.5 mL, 50 Each/Case (expiration March 2025)
930715
Skin Prep Solution ChloraPrep™ Hi-Lite Orange™ 10.5 mL Applicator 2% CHG / 70% IPA
67133
3M™ SoluPrep™ S Sterile Antiseptic Solution, 2% CHG / 70% IPA Surgical Prep, 67133, Green Tint, 10.5 mL, 50 Each/Case (expiration May 2025-June 2025)
930725
Skin Prep Solution ChloraPrep™ Scrub Teal™ 10.5 mL Applicator 2% CHG / 70% IPA
67133
3M™ SoluPrep™ S Sterile Antiseptic Solution, 2% CHG / 70% IPA Surgical Prep, 67133, Green Tint, 10.5 mL, 50 Each/Case (expiration May 2025-June 2025)
930800
Skin Prep Solution ChloraPrep™ Clear 26 mL Applicator 2% CHG / 70% IPA
67130
3M™ SoluPrep™ S Sterile Antiseptic Solution, 2% CHG / 70% IPA Surgical Prep, 67130, Green Tint, 26 mL, 20 Each/Case (expiration March 2025-August 2025)
930815
Skin Prep Solution ChloraPrep™ Hi-Lite Orange™ 26 mL Applicator 2% CHG / 70% IPA
67130
3M™ SoluPrep™ S Sterile Antiseptic Solution, 2% CHG / 70% IPA Surgical Prep, 67130, Green Tint, 26 mL, 20 Each/Case (expiration March 2025-August 2025)
930825
Skin Prep Solution ChloraPrep™ Scrub Teal™ 26 mL Applicator 2% CHG / 70% IPA
67130
3M™ SoluPrep™ S Sterile Antiseptic Solution, 2% CHG / 70% IPA Surgical Prep, 67130, Green Tint, 26 mL, 20 Each/Case (expiration March 2025-August 2025)
With any further questions you may have, please reach out to Bill Black.
Kind Regards
Communication Type:
End User Promo
Vendor #10296
Communication Type:
Product Inventory Update
Vendor #10296
-
09/13/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 09-09-2024. Also attached is a usage report for the Low Inventory Adult Institutional Items from the last 6 months.
Please note: There was no usage information for the PSN Deprioritized items or the Adult Inst. Deprioritized items from the last 6 months.
Please see the attachments listed below to reference this notification
- Abbott Institutional Inventory Letter 9_12_24
- Abbott Customer File Concordance Cross Ref.
- 09-13-2024 Abbott Weekly Inv. Update Item Usage 2
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
09/13/2024
Title: Pfizer V#12114 - Temp. Product Unavailability Update
Details:
Concordance Team,
Pfizer Product MFG#00409762003 (CHS#139717) Heparin Sodium 2u/mL .9% Sodium Chloride Injection Solution is out of Stock with an Estimated Release Date of December 2027.
An available sub has been suggested to us and is now set up in our system. MFG#00409100520 (CHS#403547) is the available sub that has been suggested.


Please see the usage file to see the affected customers.
With any further questions, please reach out to Bill Black or Mary Reinhart while Bill is at HIDA.
Kind Regards
Communication Type:
Temp Unavailable
Vendor #12114
-
09/12/2024
Title: Cardinal V#10801 - Product Availability Updates
Details:
ProductAvailabilityUpdateCAHBrand
Concordance Team,
You have received this update in the past. You will start receiving again every two weeks.
Reach out to your local Cardinal Rep with questions.
Thank you!!!
Enteral Feeding>>
Facial Protection>>
Fluid Management>>
Exam Gloves>>
Incontinence>>
Infection Control Apparel>>
Laboratory Products>>
Localized Temperature Therapy>>
Medical Essentials>>
Medicated Wipes>>
OR Accessories>>
Presource® Procedural Packs>>
Sharps Safety>>
Skin Management>>
Surgical Drapes & Gowns>>
Surgical Essentials>>
Surgical Gloves>>
Traditional & Advanced Wound Care>>
Urology>>
Women & Baby>>
Wound Drains>>
Communication Type:
Product Inventory Update
Vendor #10801
Communication Type:
Product SKU List
Vendor #10801
Communication Type:
Product Update
Vendor #10801
-
09/12/2024
Title: SC Johnson V# 12219 - Rep Spiff
Details:
Concordance_MMCAPs_Dispenser Spiff
North America Sales Force Contact List (002)TSM's
Hi Concordance Team,
SC Johnson Professional is happy to announce a Spiff for Concordance Sales Reps – Q4 2024.
This Spiff is for hand soap and hand sanitizer for your MMCAP’s customers. These are high volume products that also provide repeat sales throughout the year.
K-12
Higher Ed
Government
Other
Use this in concert with the MMCAP’s contract we have in place for Concordance.
Dispensers are provided free for your customers via POS submission on the contract.
SCJP can help with installs if needed, please contact me with guidelines.
Example:
School District ABC puts up 500 combined soap, sanitizer dispensers - you the Sales Rep will earn $2500.
Feel free to reach out to me directly with any questions or help needed.
Thanks,
Don

Items affected in the spiff are as shown below:
Vendor # Supplier MFG# CHS# Description SUOM Qty/SUOM 12219 SC Johnson GPF3LDQ 402582 SCJ Professional Heavy Duty Foam, BLACK, 3.25L Case 6/case 12219 SC Johnson 91128 402581 Proline Curve, BLACK, 1L Case 15/case 12219 SC Johnson WHB1LDS 402579 Proline Curve, WHITE, 1L Case 15/case 12219 SC Johnson WYH1LDS 402577 Kids Wash Dispenser, GREEN, 1L Case 15/case 12219 SC Johnson CLR1L 402542 Refresh Clear FOAM, 1L Case 6/case 12219 SC Johnson AZU1L 402541 Refresh Azure FOAM, 1L Case 6/case 12219 SC Johnson ANT1L 402540 Refresh AntiBac FOAM, 1L Case 6/case 12219 SC Johnson GPF3LNA 402524 Solopol GFX, 3.25L Case 2/case 12219 SC Johnson IFC1L 402569 InstantFOAM Complete PURE, 1L Case 6/case 12219 SC Johnson 55857 402538 Instant FOAM Non-Alcohol PURE, 1L Case 6/case Communication Type:
Rep Spiff
Vendor #12219
-
09/12/2024
Title: Blickman V#10660
Details:
BACK TABLES
Back Tables
Our specially designed Back Table provides critical space for the wide array of equipment and instruments necessary during larger cases when floor space is limited. Table holds multiple trays, has sanitary closed tops preventing bacterial build up, and sealed reinforcing channels which provide rigidity and durability. The top tier 'hide-away' shelf has a 6.35° tilt when in the upright position allowing for easier instrument viewing and access. Sealed casters prevent corrosion, and each holds up to 400 lbs.
Performance Pneumatic Back Table
Two Standard Sizes: 60" or 72" L x 30" D x 34" H
Height adjustment from 46"- 49"Manual Back Table
Three Standard Sizes: 48", 60" or 72" L x 30" D x 34" H
Height adjustment from 46"- 49"In-house customization is available to create the perfect size for your environment. Email us at info@blickman.com
Blickman Industries
1-800-247-5070
www.blickman.com
Copyright © 2024 Blickman Industries.
All rights reserved.
Want to change how you receive these emails?
Update your preferences or unsubscribe.Communication Type:
Product Update
Vendor #10660
-
09/11/2024
Title: Medpro - Sales Roster
Details:
MP Enterprise Sales Map - Updated August 2024
Concordance Team,
Medpro Healthcare Sales Solutions has provided us with their Enterprise Territory Map and sales roster. Please see the attached for further information.
Please reach out to Bill Black for further information.
Regards,
Communication Type:
Sales Roster
-
09/10/2024
Title: Pfizer V#12114 - Temp. Product Unavailability Notice
Details:
Concordance Team,
Pfizer Product MFG#00409762003 (CHS#139717) Heparin Sodium 2u/mL .9% Sodium Chloride Injection Solution is out of Stock with an Estimated Release Date of December 2027.
Please see the usage file to see the affected customers.
With any further questions, please reach out to Bill Black or Mary Reinhart while Bill is at HIDA.
Kind Regards
Communication Type:
Temp Unavailable
Vendor #12114
-
09/9/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_6Sep2024
Nestle Weekly Inv. Update Cross Ref.
09-10-2024 Nestle Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 08-19-2024. The following changes are being made:
- Peptamen Intense 1000 ml was removed
- Alfamino Infant was removed
- Fibersource HN 250 ml was added
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
09/9/2024
Title: Abbott V#10575 - Product Availability Update
Details:
09-11-2024 Abbott Product Availability Update Item Usage
Concordance Team,
Abbott Nutrition is providing an update that the following SKUs are available to order. These items will have quantities reviewed to ensure equitable access for patients across all channels.
Effective 09/10/2024, the below items will become available to order:
MFG# VAI Item Item Description 64905 248663 Ensure Plus Van 8oz ARC 64929 248675 Glucerna Shake Choc 8oz ARC 64907 248662 Ensure Plus Straw 8oz ARC With any further questions you may have, please reach out to Mary Reinhart.
Kind Regards,
Communication Type:
Product Inventory Update
Vendor #10575
Communication Type:
Product Update
Vendor #10575
-
09/6/2024
Title: Nestle V#10183 - Product Relaunch
Details:
NHSc Customer Communication Vivonex Powder Letter 09.01.24 MS W Blue
Letter Vivonex Ped Cross Reference
Spec Sheet Vivonex Pediatric Powder Canister 08.08.2024
09-10-2024 Vivonex Ped. Unflavored Packet Item Usage
Concordance Team,
Nestle is relaunching Vivonex Pediatric Elemental Powder in a new and improved canister with an easy-to-store scoop rather than the single-serve packets. There are some improvements made to the product as well.
1. Now also indicated for Chylous Ascites and Chylothorax
2. Now hypoallergenic
3. Now certified Halal
What Is Changing?
What Is NOT Changing?
· Servings/case goes from 36 to 49.5
· Price per case adjusted due to
increased calories per case
· Packaging
· UPCs, Product Codes and NDC-format numbers
· Micronutrient changes to reflect current Dietary Reference Intakes (DRIs)
· Case/pallet dimensions
· Now Halal certified
· No longer Kosher certified
· Price per calorie
· Macronutrient profile
· HCPCS code: B4161
· Shelf life: 24 months
Please see the attachments above for further information.
With any further questions you may have, please reach out to Mary Reinhart.
Kind Regards,
Communication Type:
New Product Launch
Vendor #10183
Communication Type:
Product Update
Vendor #10183
-
09/5/2024
Title: Neogen V#13577
Details:
Thank you so much for the opportunity to discuss Neogen® CleanTrace™ ATP Monitoring System with you. This is a quantitative cleaning verification tool that can provide you with the real time data information. It is very intuitive to the end users and easy to navigate. The Quality Control Data Manager (QCDM) is one of the most robust and easy to customize in the industry. We can also provide the Application Program Interface (API) our customer who would prefer to conduct their own data analytic program.
The attachments are some of the general product materials. I will be working on getting you a contact from one of our customers so, you can get the direct feedback on Neogen® Clean-Trace™ ATP Monitoring System.
Please feel free to contact me should you have any additional questions. We are looking forward to partnering with you and team. Thank you.
Best regards,
Keith K. Liansi, CSPDT, C.F.E.R.
Key Account Manager | Healthcare
Mobile: 347.385.7403
Phone 517.334.0821 ext. 55445
Tech Support:1.800.234.5333
Press 7 for US technical assistance.
Press 2 for Assistance with readers.
Press 2 for LX25
Email: kliansi@neogen.com
Communication Type:
Product Flyer
Vendor #13577
Communication Type:
Product Update
Vendor #13577
-
09/5/2024
Title: Attends V#10055
Details:
Please see attached Product now available- Attends DDS Stretch Brief Bi-Fold for Nursing Homes
Please reach out to Heather Burkett with any questions. Thanks,
Communication Type:
New Product Launch
Vendor #10055
Communication Type:
Product Flyer
Vendor #10055
-
09/5/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 09-05-2024. Also attached is a usage report for the Adult Institutional Items from the last 6 months.
Please note: There was no usage information for the PSN Deprioritized items or the Adult Inst. Deprioritized items from the last 6 months.
Please see the attachments listed below to reference this notification
- Abbott Institutional Inventory Letter 9_5_24
- Customer File Concordance Cross Ref. 9-5-24
- 09-10-2024 Weekly Inv. Update Item Usage
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
09/4/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_30Aug2024
Nestle Inv. Update Item Cross Ref.09-03-2024
Nestle Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 08-19-2024. The following changes are being made:
- No Changes to note.
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
09/4/2024
Title: MOOG V#10244 - Product Discontinuation Notice
Details:
ENFit Transitional Connector Discontinued_2024_06_15_
Moog Product Discontinuation Item Cross Ref.
09-04-2024 Moog Product Discontinuation Item Usage
Concordance Team,
MOOG has let us know they fully support the phasing out of legacy enteral feeding products and the transition to ENFit Connectors. All current MOOG products are ENFit-only compatible.
During the transition, and in order to support patients with non -ENFit feeding tubes, MOOG has sold separately packaged ENFit Transitional Connectors (FOOO71). These transitional connectors were planned to be available for a limited time only. In accordance with that plan, the ENFit transitional connectors (F00071) will be available for sale through September 15, 2024 or until inventory is sold out, whichever comes first.
If you have any further questions, please reach out to Mary Reinhart.
Best Regards
Communication Type:
Discontinuation Notice
Vendor #10244
-
09/4/2024
Title: BD V#10125 - Product Availability Update/ Manual BD Allocation
Details:
BD ChloraPrep 10.5ml and 26ml supply update for BD customers 9.02.24
09-04-2024 BD Chloraprep Item Usage
Concordance Team,
Due to increase customer demand over the past 6months, BD ChloraPrep 10.5mL adn 26mL Single Sterile Applicators will be placed on manual order review to ensure BD can maximize product availability for all BD customers. We have not requested the products to go on external allocation with their channel partners as we anticipate meeting customer demand during this time.
The products listed in Table 1 below may experience short0term delays in order fulfillment through October 2024. There is no interruption in BD ChloraPrep manufacturing operations with these product codes and this action will help BD better support our needs.
Table 1: List of Impacted Products
MFG# CHS# Description 930700 317174 BD ChloraPrep Clear 10.5mL Applicator 930715 317175 BD ChloraPrep Hi-Lite Orange 10.5mL Applicator 930725 317187 BD ChloraPrep Scrub Teal 10.5mL Applicator 930800 317189 BD ChloraPrep Clear 26mL Applicator 930815 314903 BD ChloraPrep Hi-Lite Orange 26mL Applicator 930825 317195 BD ChloraPrep Scrub Teal 26mL Applicator Recommended Actions:
1. We ask that you continue to order your BD ChloraPrep 10.5mL and 26mL Single Sterile Applicators per historical volumes. Significant orders may be subject to manual order review and partial shipment with excess volumes cancelled.
2. To ensure supply continuity we ask customers to place orders commensurate with normal utilizations this will help BD Minimize the impact to the market.
3. Please do not pull forward orders or place stockpile orders.
4. Orders will continue to be shipped based on customers' historical monthly average ordering patterns.
Next Steps:
1. We will continue to release orders FIFO (First in First out) but will reviewing inventory on hand each day to support critical needs.
2. Releases will be light the week of September 2nd and inventory begins improving the week of September 9th. If there are urgent customer needs next week and your customers are in short supply, your customer want to consider purchasing a week’s worth of an alternative solution until releases are improved the following week. Here are some possible alternatives depending on each institution’s individual needs:

If you have any further questions, please reach out to Bill Black.
Best Regards
Communication Type:
Allocation Notification/Update
Vendor #10125
Communication Type:
Product Inventory Update
Vendor #10125
-
09/4/2024
Title: Jant V#10420 - Product Recall Notice
Details:
09-04-2024 Jant Product Recall Item Usage
Concordance Team,
Please see the attachments for further information on a product recall from Jant Pharmacal Corp. V#10420. This recall impacts the following products:
MFG# VAI Item UA774 381670 UA710B 381665 UA824B 381666 UA711 381667 UA924 381668 With any further information, please reach out to Mary Reinhart.
Best Regards
Communication Type:
Recall Notification
Vendor #10420
-
08/30/2024
Title: Health-O-MeterV#10075
Details:
BABY SCALES sales sheet
Health o meter Professional Announces New High-Resolution Digital Baby Scale
Pelstar LLC, the manufacturer of Health o meter® Professional Scales, is proud to introduce the 565KL neonatal/pediatric scale specifically designed to meet the rigorous demands of healthcare professionals in critical environments such as NICUs, nurseries, delivery rooms, and pediatric practices. This state-of-the-art scale ensures exceptional accuracy, crucial when caring for the tiniest patients.
The 565KL features a gently curved cradle that provides maximum comfort for infants and a large, angled display that allows caregivers to easily read weight measurements. Adjustable feet ensure a level weighing surface, and a built-in measuring tape enables simultaneous weight and height assessment. Additionally, there is an option for a digital height rod (565EHR). Even with active infants, enhanced motion detection technology ensures quick and accurate readings. With a high 1-gram resolution and a capacity of 20 kg (44 lb), this scale is precise and versatile. Features include reweighing, hold/release, zero, recall, five-minute weight hold, wireless capabilities, and more. A kilogram-only model is also available. For additional product information, visit https://www.homscales.com/565kl or call 1-800-815-6615.
thanks,
Kelly Virzi
Sales and Marketing Specialist
Pelstar LLC/Health o meter® Professional Scales
9500 West 55th Street
Communication Type:
Product Flyer
Vendor #10075
-
08/30/2024
Title: Sekisui V#10161 - hCG Discontinuation Notice
Details:
Sekisui Product Discontinuation CHS Cross Ref.
08-30-2024 Sekisui Product Discontinuation Item Usage
Concordance Team,
Due to rising manufacturing costs, Sekisui has regretfully informed us of their intent to modify their current hCG portfolio offerings. As an existing customer of the OSOM hCH Urine Test (part #101), OSOM hCG Card Pregnancy Test (part #102w), and/or OSOM hCG Combo Test (part#124), we are informing you that we intend to discontinue the following products effective March 3, 2025:
DISCONTINUED PRODUCTS REPLACEMENT PRODUCT Part Number CHS# Item Name Description Sample Type Item Name & Part Number CHS# Description Sample Type 101 333621 OSOM hCG Urine Test 50 hCG Urine test sticks Urine #1004 OSOM UltrahCG Combo Test 232331 25 Ultra hCG Combo test devices Urine & Serum 102W N/A OSOM hCG Card Pregnancy Test 25 hCG Card test devices Uring 124 131056 OSOM hCG Combo Test 25 hCG Combo test devices Urine & Serum For further information, please reach out to Bill Black.
Kind Regards
Communication Type:
Discontinuation Notice
Vendor #10161
-
08/28/2024
Title: Hemocue V#10452 - Promotions, Rep Roster & Costing for Promotions
Details:
HemoCue Field Sales Roster 8-21-2024
08-27-2024 Hemocue Promo Item Usage
Concordance Team,
Please see the attachments for all promotions, rep roster and Item usage from the last 6 months. In the chart below details the costing on the promotions. The first price is your cost and the second is the retail price.
PROMOTIONAL COMBINATION PACKS Product ordered and drop shipped directly from HemoCue G1DMPROMO HemoCue Glucose DM System - 1Box $1,409.00 $1,761.00 (Perishable product;must be refrigerated; non-returnable) G1PROMO HemoCue Glucose 201 System Starter - 1 Box $248.00 $314.00 (Perishable product;must be refrigerated; non-returnable) G3PROMO HemoCue Glucose 201 System Starter - 1 Box Discontinued Discontinued (Perishable product; must be refrigerated; non-returnable) GDMPPROMO HemoCue Glucose DM with Dock $1,534.00 $1,918.00 H1DMPROMO HemoCue Hb DM System - 1 Box $1,321.00 $1,650.00 H1PROMO HemoCue Hb 201+ System Starter - 1 Box Discontinued Discontinued HB1PROMO HemoCue Hb 801 Analyzer +200 cuvettes $292.00 $389.00 HB3PROMO HemoCue Hb 801 Analyzer +600 cuvettes Discontinued Discontinued HDMPPROMO HemoCue Hb DM with Dock $1,511.00 $1,888.00 With any further questions you may have, please reach out to Bill Black.
Kind regards,
Communication Type:
End User Promo
Vendor #10452
Communication Type:
Sales Roster
Vendor #10452
-
08/28/2024
Title: BD V#10125
Details:
Dear Valued BD BACTEC™ Blood Culture System User,
We are pleased to report that we are making strong progress to restore supply of BD BACTEC™ blood culture vials. Through our efforts to improve output with our supplier, improve production line efficiency, and encourage temporary use of glass vials, we expect to be back to meeting 100% of historic weekly demand volumes for the U.S. market by the end of September. The replenishment of our distribution partner’s warehouses is our top priority and inventory levels continue to improve.
The latest information is available on BDBACTEC-update.com. This enhanced website is designed to keep customers informed of progress in rebuilding supply with links to helpful resources, including information about the BD BACTEC™ Lytic/10 Anaerobic/F Culture Vials in glass, which will be available in the U.S. beginning in early September. Information is also available on the interim shelf-life extension for specific lots of BD BACTEC™ blood culture vials. (A list of lot numbers is available under “Resources” on the website.)
We’ve established a hotline (1.888.834.4332) to support any questions our customers may have and provide resolution. An opt-in notification option is now available for anyone who wants to receive email updates.
Please visit BDBACTEC-update.com for the latest information. As always, please contact your BD representative at any time with questions or immediate needs.
Sincerely,
BD BACTEC™ teamCommunication Type:
Product Inventory Update
Vendor #10125
Communication Type:
Allocation Notification/Update
Vendor #10125
-
08/28/2024
Title: BD V#10125 - Supply Update for Planned Product Discontinuation
Details:
MMS ISD BD-35964QQ 2420-0500 Request of Immidiate Migration 8-23-24
08-27-2024 BD (V#10125) Short Term BO Item Usage
Concordance Team,
Excel file attached is usage so you can reach out to your customer.
- Communication letter requesting migration from 2420-0500 to 2420-0007 and from 2426-0500 to 2426-0007 immediately
- (2420-0500 CHS # 442509) Move to 2420-0007 CHS # 131129
- (2426-0500 CHS # 517938) Move to 2426-0007 CHS # 170206
Please see the information from BD below:
- Communication letter requesting migration from 2420-0500 to 2420-0007 and from 2426-0500 to 2426-0007 immediately
Supply update for planned product discontinuation
Dear Concordance team,
We received notification from a third-party supplier of a short-term raw material disruption impacting specific Alaris LVP Pump sets (2420-0500 and 2426-0500), which will result in short-term BO for these sets. The identified risk only impacts the 2420-0500 and 2426-0500 products.
These products were set for discontinuation by the end of the calendar year, refer to customer notification sent in July, (BD-35964NN dated July 10, 2024), with transition to analogous alternatives that do not contain DEHP as part of the material formulation (see tables below).
To ensure supply continuity, we are requesting immediate transition to the alternative products: 2420-0007 and 2426-0007. Early migration will help to mitigate much of the supply disruption; however, some BO on these sets may occur. BD expects the raw material issue to be resolved in early September and inventory positions to stabilize by September end/early October.
Learn more about the 2420-0500 and 2426-0500 migrations in the letter attached and accessing:
- All you need to know in one place – BD mini-website: www.bd.com/IPDportfolio
- How can the optimized portfolio improve your efficiency – Video: IPD Portfolio Mix Shift_Customer Video (bd.com)
Sincerely,
Heather
Heather Greve
Channel Management Acute VP
Indiana – FIELD
Indianapolis, IN 46143
US
c: 317.523.7526
awb://bd.com
Communication Type:
Discontinuation Notice
Vendor #10125
Communication Type:
Product Update
Vendor #10125
-
08/27/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 08-26-2024. Also attached is a usage report for the Adult Institutional Items from the last 6 months.
Please note: There was no usage information for the PSN Deprioritized items or the Adult Inst. Deprioritized items from the last 6 months.
Please see the attachments listed below to reference this notification
- Abbott Institutional Inventory Letter 8_22_24
- Customer File 8-22-24 Concordance Cross Ref.
- 08-27-2024 Abbott Weekly Inv. Update Item Usage
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
08/26/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_23Aug2024
Nestle Weekly Inv. Update Item Cross Ref.
08-26-2024 - Nestle Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 08-19-2024. The following changes are being made:
- Alfamino Infant was added
- Nutren 1.5 1000 ml was added
- Peptamen PreBio 250 ml was added
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
08/23/2024
Title: Attends V#10055
Details:
New product available- DD70 from Attends. Please click here for pricing and more info. Reach out to Heather Burkett if you have any questions. Thanks,

Communication Type:
New Product Launch
Vendor #10055
-
08/22/2024
Title: Mead Johnson V#10502
Details:
Tornado/Paused items
Due to the Tornado that struck a Mead Johnson warehouse, we have had to mark some items TEMP UNAV to order at this time. My contact thinks this should last through the end of September. Attached is a letter from Mead along with customer usage. All items that were paused are on the HFS tab of the price list that is also attached. I will post these in HUBSPOT for future reference and needs.
We will working with Mead to get the most up to date inventory reports as they are released.
Please let me know if you have any questions, always happy to assist.
Have a great day!
Communication Type:
Temp Unavailable
Vendor #10502
-
08/22/2024
Title: Sempermed V#10078
Details:
TTNF Product disruption
We are being alerted to a possible temporary disruption in service from Sempermed on their series TTNF Gloves. Disruption should only be until the end of September. I have attached 6 month usage of the affected gloves as well as DOH per warehouse. Our DC’s with less than 30 DOH may experience stock outs.
HCS is listed as a sub to these items, so please check with the sourcing team.
CHS#’s for HCS gloves
221759
221760
221761
221863
Also attached is a flyer for the suggested sub, SMTN series gloves from Sempermed. Same glove, just comes in a box of 250 instead of 200.
CHS# for STMN gloves
210739
210740
210741
210742
210743
Please reach out to me for any questions or concerns you may have.
Have a great day!
--------------------------------------------------
Mary Reinhart | Supplier Relations, Manager
Concordance Healthcare Solutions
85 Shaffer Park Drive, Tiffin, OH 44883
419-455-2162 | mreinhart@concordancehs.comCommunication Type:
Product Inventory Update
Vendor#10078
-
08/22/2024
Title: Greiner V#11403
Details:
EVOPROTECT PRODUCT LAUNCH
Good Morning Greiner Bio-One Distribution Partners,
We recently sent an email blast to GBO customers regarding our EvoProtect line of semi-automatic butterfly needles. We have received numerous requests for a solution to a competitor's product with supply issues. We then received requests for this information from local distribution sales staff. I am attaching the information that contains links to specific product details with the request that you share this information with our sales teams. These products are readily available and priced according on your pricing schedules.
Please share the attached document with your sales teams and let me know if you have any questions. There is also a link in the attached flyer to request additional information or to schedule a product demo.
Thank you,
Terry Prymek
________________________________________________________
Terry Prymek
Key Account ManagerGreiner Bio-One North America, Inc.
4238 Capital Drive · Monroe · NC · 28110
M: 704-254-6211
Terry.Prymek@gbo.com · https://url.avanan.click/v2/___www.gbo.com___.YXAzOmNvbmNvcmRhbmNlaHM6YTpvOjcxZTNlZDliY2MxOTNjODg3NGMwNTFhYjhiMzYwN2QzOjY6ZWI5ZTpjNWY3ZTUzYWViMzVjYWU0ZmUyNzg1MGRmNDZiNzc4YWFlZDk3YjhmNDE2Mzg4ODk3NTQyZDhkMzc3YjNjMWViOnQ6RjpO
Communication Type:
Product Flyer
Vendor # 11403
-
08/20/2024
Title: Airlife V#11179 - CAPNO SKU Update
Details:
Concordance Team,
Please see the notice attached for further information on Airlife (V#11179) CAPNO SKU change.
With any further questions you may have, please reach out to Jeff Shook.
Thank you!
Communication Type:
Product Changes
Vendor #11179
-
08/20/2024
Title: Attends V#10055
Details:
Booster Pads- Off Backorder/ Packaging Change
As we being to ship again the Attends Booster Pads BST0192 they will be in White Bags then transition into its final Attends Updated Packaging.
The following PDF explains the transition process below.

Communication Type:
Backorder Notification/Update/Reports
Vendor #10055
Communication Type:
Product Changes
Vendor #10055
Communication Type:
Label Changes
Vendor #10055
-
08/19/2024
Title: Attends V#10055
Details:
Attends Product Tiers- August 2024
Please see attached for update from Attends. If you have any questions please reach out to Heather Burkett. Thanks,
Communication Type:
Product Catalog
Vendor #10055
Communication Type:
Product Flyer
Vendor #10055
-
08/19/2024
Title: Attends V#10055
Details:
Attends Gender-specific Underwear for Women and Men
Please click here for PDF. The New Attends Men’s XLG Underwear - ADUM45 has been added to this PDF. If you have any questions please feel free to reach out to Heather Burkett. Thanks,
Communication Type:
Product Flyer
Vendor #10055
Communication Type:
New Product Launch
Vendor #10055
-
08/19/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 08-12-2024. Also attached is a usage report for the Adult Institutional Items from the last 6 months.
Please note: There was no usage information for the PSN Deprioritized items or the Adult Inst. Deprioritized from the last 6 months.
Please see the attachments listed below to reference this notification
- Abbott Institutional Inventory Letter 8_15_24
- CustomerNum File 8-15-24 Concordance Cross Ref.
- 08-19-2024 Abbott Weekly Inv. Item Usage
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
08/19/2024
Title: Abbott V#10575
Details:
Product discontinuance
Abbott has decided to discontinue PediaSure 1.5Cal with Fiber Ready to Hang (Abbot Item #67376). The discontinuation of this item is effective immediately. Additional information, including product alternatives, can be found within the attached letter.
If you have any questions, please contact a Distributor Relations team member at the contact information below.
Abbott Nutrition Distributor Relations Team Contact Information:
Nick Saffell; Sr. Business Analyst: email: Nicholas.Saffell@abbott.com; phone: 614‐624‐6446
Joe Corey; Sr. Business Analyst: email: joe.corey@abbott.com; phone: 614‐624‐6600
Judi Troutman; Manager: email: Judi.Troutman@abbott.com; phone: 614‐624‐3896
Thank you,
Joe
Communication Type:
Discontinuation Notice
Vendor #10575
-
08/19/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_16Aug2024
Weekly Inv. Update CHS Cross Ref.
08-20-2024 Nestle Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 08-12-2024. The following changes are being made:
- Compleat pediatric Standard 1.0 was added
- Diabetisource AV 1500 ml was added
- Novasouce Renal 1000 ml was added
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
08/16/2024
Title: Hartmann V#10739
Details:
Hartmann disco item list_usage 2.16.24-8.15.24
Zetuvit Plus Silicone Non-Border Launch July24
Concordance Team,
Please see the attached letter from Hartmann about 5 item numbers that are being DIRECTLY REPLACED with 5 new item numbers. As stated in the letter, "There are no changes to the product itself. Why the change? The new item numbers feature the same product in smaller more streamlined pouches which also allows for smaller box dimensions. The boxes feature new branding as well as a QR code to quickly view application videos."
Regards,
Tricia Wentling
Communication Type:
Discontinuation Notice
Vendor # 10739
Communication Type:
New Product Launch
Vendor # 10739
-
08/16/2024
Title: Steris V#10863
Details:
Steris disco item CHS # 901181_usage 2.16.24-8.15.24
Concordance Team,
SEE BELOW MESSAGE FROM THE SUPPLIER:
This letter is to inform you that effective September 1, 2024, STERIS will no longer accept orders for the following product:
SKU NM410
Description: STAINLESS STEEL CLNR 15OZ (425 G) AEROSOL CANRecommended alternative product for the stainless steel cleaner:
SKU: CL841
Description: STAINLESS STEEL CLEANER (15 OZ AEROSOL CAN)
Selling UOM: EA
Distributor Acquisition Cost: $10.00/EA
Best regards,
Tricia Wentling
Communication Type:
Discontinuation Notice
Vendor #10863
-
08/15/2024
Title: ICU Medical V#10642 - Product Discontinuation Notice
Details:
P24-7026 TranStar to Transpac Conversion Ltr - Distribution
Concordance Team,
Effective December 31, 2024 the following products will be discontinued.
The letter attached is to inform you about a transition in ICU Medical's product offering. In an effort to streamline our product portfolio and enhance overall customer experience, ICU Medical will be consolidating their transducer line. As part of this consolidation effort, ICU Medical will be sunsetting the TranStar product line, fulfilling orders for as long as inventory is available. The Transpac product line will then become the primary solution for your blood pressure monitoring needs. End-users can work with their field representative to assist with a transition plan to the Transpac line.
The following codes will be discontinued:
Item Number Item Description


Please see the attachment for further information. With any further questions, please reach out to Bill Black.
Regards
Communication Type:
Discontinuation Notice
Vendor #10642
-
08/15/2024
Title: Convatec V#10695 - recall notice
Details:
Certificate of Destruction - TW-1939942_English
Recall Notice - Eakin_APPROVED
Concordance Team,
MESSAGE FROM CONVATEC:
On behalf of the legal manufacturer, TG Eakin Ltd, Convatec Ltd is formally cascading a recall notification regarding Cohesive Small Seals / Product Code: 839002 to customers identified as affected.
The attached recall notice contains all the relevant information including, instructions on the immediate actions required of the consignee. Please ensure this communication is shared with the correct department, function, or individual within your organisation i.e., Compliance Team, Regulatory Team, Recall Team etc.
On behalf of TG Eakin Ltd, Convatec would like to apologise for any inconvenience caused by this action.
The relevant regulatory agency was notified of this event on 09-Aug-24.
Regards,
Tricia Wentling
Regards,
Communication Type:
Recall Notification
Vendor #10695
-
08/13/2024
Title: Essity V#10272
Details:
Discontinuation (2022)/ Replacement Item
Please see attached for new item that replaces C#218909 that was discontinued in 2022.
If you have any questions, please reach out to Heather Burkett. Thanks,
Communication Type:
Discontinuation Notice
Vendor #10272
Communication Type:
New Product Launch
Vendor #10272
-
08/12/2024
Title: Cardinal V#10801 - Product Discontinuation Notice
Details:
May 2024 Portfolio Update + Rationalization Customer Letter
Concordance Team,
For further information on the topics mentioned below, please see the Customer Letter attached.
Full portfolio discontinuation:
As a part of this work, we will discontinue our Cardinal Health™ Grooming, Baby Care Grooming, Lube Jelly, Feminine Hygiene, and Infant CPAP portfolios in July 2024, or when inventory is fully depleted.Portfolio reduction only:
As we simplify additional Cardinal Health brand businesses, we will discontinue select products within the portfolios listed below. When inventory is depleted, you will need to transition your ordering to a Cardinal Health™ product or alternate supplier.- Abdominal Belts - Abdominal Binders
- Baby Wipes - Clinical Exam - Open Suction - Paper Products - Skin Management - Slippers - Sterilization Wrap - Surgical Drapes - Wound Drains With any further questions you may have, please reach out to Bill Black.
Best Regards
Communication Type:
Discontinuation Notice
Vendor #10801
-
08/12/2024
Title: Thomas Medical V#10463 - Product Information Update
Details:
Concordance Team,
Please see the attached for further information on Thomas Medical's Electrodes.
With any further questions you may have, please reach out to Mary Reinhart.
Best Regards,
Communication Type:
Product Flyer
Vendor #10463
-
08/12/2024
Title: Solventum V#10296 -
Details:
08-14-2024 Solventum Littman MAP Item Usage
Concordance Team,
Please see the notice below from Solventum PKA 3M Healthcare V#10296.
Dear Customer,
We are pleased to announce “2024 September Back to School MAP” by lowering MAP on all Covered Products from September 1 to 30, 2024*.
Please note the MAP Policy remains in effect during this time, and the only change is the lowered MAP on Covered Products.
Please see the September MAP highlighted in the following table:
Material Cat# CHS# Billing Unit 2024 MAP 2024 September Back to School MAP Sept 1-30, 2024 3M™ Littmann® CORE Stethoscope, 8480, Black 8480 332854 Each $329.00 $299.00 3M™ Littmann® CORE Stethoscope, 8570, HP Rainbow 8570 357859 Each $349.00 $319.00 3M™ Littmann® CORE Stethoscope, 8870, HP Copper 8870 #N/A Each $349.00 $319.00 3M™ Littmann® CORE Stethoscope, 8890, Mirror 8890 #N/A Each $349.00 $319.00 3M™ Littmann® CORE Stethoscope, 8483, Black, Eko 8483 #N/A Each $329.00 $299.00 3M™ Littmann® CORE Stethoscope, 8574, HP Rainbow, Eko 8574 #N/A Each $349.00 $319.00 3M™ Littmann® CORE Stethoscope, 8838, HP Copper, Eko 8838 #N/A Each $349.00 $319.00 3M™ Littmann® CORE Stethoscope, 8839, Mirror, Eko 8839 #N/A Each $349.00 $319.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Black Tube, 22 inch, 6151 6151 235533 Each $199.00 $184.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Black Tube, 27 inch, 6152 6152 235537 Each $199.00 $184.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Navy Blue Tube, 27 inch, 6154 6154 235540 Each $199.00 $184.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Hunter Green Tube, 27 inch, 6155 6155 235541 Each $199.00 $184.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Plum Tube, 27 inch, 6156 6156 235542 Each $199.00 $184.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Raspberry Tube, 27 inch, 6158 6158 235544 Each $199.00 $184.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Rose Pink Tube, 27 inch, 6159 6159 242502 Each $199.00 $184.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Burgundy Tube, 27 inch, 6184 6184 #N/A Each $199.00 $184.00 Please be advised that this MAP Policy concerns only certain advertised prices and does not relate to the actual sales prices of any product.
*2024 September Back to School MAP will start on September 1, 2024 at 12:01 a.m. (EST) and end on September 30, 2024 at 11:59 p.m. (EST).
MAP Policy Update
Please be informed that Solventum has updated the Minimum Advertised Price Policy, effective September 1, 2024 to reflect the company change from 3M Company to Solventum Corporation and the notification period for changes on MAP of any Covered Product is updated as 10 business days.
“Solventum may update, revise, suspend, terminate, reinstitute, or modify this Policy at any time in its sole discretion. Solventum shall make any such modifications available to all Solventum’s reseller customers. If Solventum changes the MAP on any Covered Product, it will provide 10 business days’ notice to such reseller customers before such change takes effect. No Solventum employee or agent, including a reseller’s sales representative, is authorized to modify, interpret, or grant exceptions to this Policy; solicit or obtain the agreement of any person to this Policy; or otherwise discuss any aspect of this Policy with any reseller, including that reseller’s or any other reseller’s compliance with the terms of the Policy.”
A copy of the updated Policy is attached.
Thank you,
3M Littmann MAP Administrator
With any further questions you may have, please reach out to Bill Black.
Best Regards
Communication Type:
End User Promo
Vendor #10296
-
08/12/2024
Title: Kimberly Clark V#10765 - Kimberly Clark Digital Room
Details:
Concordance Team,
Please see the email and link distributed by Bill Black below. With any further questions, please reach out to Bill Black.
I wanted to share with you a link to the Concordance Healthcare Digital Room, which is a valuable resource for your sales team. This room contains a wealth of information about KCP, including contact information, catalogs, promos, new product information, and segment bundle information. Please feel free to share this link with your team.
Updated Digital Sales Room <- this is an updated sales room link - please share this link with your sales team vs the other one!
If you or your team have any questions or need assistance with contracts, promos, or general information, please don't hesitate to reach out to me. I am always happy to help!
Thank you for your time, and I look forward to working with you and your team.
Best,
Brandt Winters
Digital Sales Account Manager
Greater Lakes Ohio Valley Region
B 470-400-8856 | Lets Connect on Your Time
brandt.winters@kcc.com | kcprofessional.com | https://www.linkedin.com/in/brandt-winters-2b9260163/
1400 Holcomb Bridge Road, Roswell, GA 30076
Regards,
Communication Type:
Product Catalog
Vendor #10765
Communication Type:
Updated Supplier Information
Vendor #10765
-
08/9/2024
Title: Dukal V#10239 - Back in stock notice
Details:
2024.11.03_ProductDelay_Toothpaste_RinseFree
Concordance Team,
The items in the attached file have been removed from Temp Unav status. They are in stock and shipping again.
Thank you.Communication Type:
Product Inventory Update
Dukal V#10239
-
08/8/2024
Title: BSN V#10231
Details:
Product Announcement- New and Discontinued Items
Please see attached and below for discontinuation on C#335924 and C#374844 from BSN Medical and Marathon Medical. Also a new product could possibly be an alternative for the discontinued item. Thanks,
Communication Type:
Discontinuation Notice
Vendor #10231
Communication Type:
New Product Launch
Vendor #10231
-
08/8/2024
Title: BD V#10125
Details:
Discontinuations from BD- Please see below and attachments. Thanks,
Concordance team,
Across BD, we routinely evaluate our product portfolio to ensure we are continuing to meet the needs of our customers – and patients – around the globe. As a result, we will be making strategic decisions affecting our product portfolio. We believe these decisions can offer you a heightened customer experience by increasing the predictability of supply and demand, as well as the speed at which we can deliver products to you. In addition, we believe this will enable the introduction of new, innovative products.
To support your own planning, we want to give you notice of the following product changes. Below is a list of upcoming discontinuations - please refer to the appendix for the specific list of impacted products purchased by your organization, the discontinuation timeline, and recommended alternate BD products.* You and others in your organization may also receive additional information from BD about these upcoming discontinuations.
Material
Discontinued Date
4 Alaris Medley LVP products
1 Alaris Plus product
5 Gravity Sets products 1
5 Gravity Sets products
1 Gravity Set product
4 Intravenous Access Extension Sets & Accessories
1 Hazardous Drug Safety
03/31/2025
03/31/2025
Immediately
03/31/2025
05/31/2025
03/31/2025
03/31/2025
Pyxis™ PARx system ²
09/30/2029
Product from the Peripheral portfolio
03/31/2025
Abramson Sump Drains 3
Select products from the Temperature Sensing Foley Catheter Strips & Trays 4
8/31/2024
Medical Equipment Roll Stand 5
Immediately
Brother printer paper (20 sheet cartridge)
08/31/2024
Various BBL™ Sensi-Disc products
One BD Difco™ Antiserum Product 6
01/01/2025
07/31/2024
1 - Production restrictions due to absence of set components for these group of SKUs
2 - Pyxis™ PARx software versions 5.0 and 5.1 have previously been announced End of Life and have achieved End of Support.3 - Supplier no longer able to provide necessary component
4 - Alternative products available
5 - Identical SKU available
6 - BD Difco™ Antiserum product will be discontinued due to technical challenges affecting our ability to manufacture.
BD appreciates and values your business and looks forward to your continued interest in our products. While we recognize this change may impact your organization, we are confident that any potential short-term impact will be offset by our commitment to providing innovative products, solutions, and services.
To best serve you, all inquiries and requests should be directed through your local sales representatives or by contacting BD Customer Service at 1-844-823-5433.
Communication Type:
Discontinuation Notice
Vendor #10125
-
08/8/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_2Aug2024
Nestle Weekly Inv. Update Cross Ref.
08-08-2024 Nestle Weekly Inv. Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 07-29-2024. The following changes are being made:
- Boost VHC Chocolate was added
- Diabetisource AC 1500 ml was removed
- Novasource Renal 1000 ml was removed
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
08/8/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 08-05-2024. Also attached is a usage report for the Adult Institutional Items from the last 6 months.
Please note: There was no usage information for the PSN Deprioritized items or the Adult Inst. Deprioritized from the last 6 months.
Please see the attachments listed below to reference this notification
- Abbott Institutional Inventory Letter 8_8_24
- Customer File 8-8-24 Concordance Cross Ref.
- 08-09-2024 Abbott Weekly Inv. Update Item Usage
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
08/6/2024
Title: Midmark V#10987 - Next Generation Durability Product Information
Details:
Concordance Team,
Midmark has provided a Selling Guide and Product Info.
When your customers invest in the next generation of Midmark Steam Sterilizers, you can be confident they are getting the best. Midmark sterilizers are the market leaders year after year.
Reduced Maintenance Cost
Next generation Midmark sterilizers have been designed for durability, including an increased device life of 25,000 cycles thanks to a completely reengineered chamber. This can reduce the frequency o required maintenance by users and certified technicians by more than half.
Reduce Lifetime Cost
Device users save thousands more on operating and device care costs compared to competitive models.
If you have any further questions, please reach out to Bill Black.
Regards,
Communication Type:
Product Catalog
Vendor #10987
Communication Type:
Product Flyer
Vendor #10987
-
08/5/2024
Title: Nestle V#10183 - Discontinuation notice
Details:
BOOST Plus® vs BOOST® VHC Side-by-Side 71124
BOOST® High Protein vs BOOST Plus® vs BOOST® VHC Side-by-Side 071124
Copy of Disco ProductCross Reference 07.23.2024
Nestle discontinuation item usage 2.1.24-8.1.24
Nestle Health Science Distributor Communication Letter 8.1.24
Nutren® 1.0 Fiber vs Fibersource® HN Side-by-Side 70924
Concordance Team,
Please see the list of discontinued items from Nestle. Contact Mary Reinhart if you have any questions.
Thank you.
Communication Type:
Discontinuation Notice
Vendor #10183
-
Communication Type:
Updated Supplier Information
Vendor #10863
Communication Type:
Product Update
Vendor #10863
-
08/2/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 07-29-2024. Also attached is a usage report for the Adult Institutional Items from the last 6 months.
Please note: There was no usage information for the PSN Deprioritized items or the Adult Inst. Deprioritized from the last 6 months.
Please see the attachments listed below to reference this notification
- Abbott Institutional Inventory Letter 8_1_24
- Customer File 8-1-24 Concordance Cross Ref.
- 08-02-2024 Abbott Weekly Inv. Update Item Usage
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
08/1/2024
Title: Convatec V#10695
Details:
Discontinued item
Please see attachments regarding Convatec disco/replace. Ease strips (422163) have been discontinued and replaced by Esenta strips (423826) as of August 1. Please let me know if you have any questions. This product is primarily sold through DME’s so it’s low volume for Concordance.
- Disco/replace letter
- PDAC letter
Best regards,
Jim
Jim Soares
Director, Channel Sales
Convatec, USA
200 Crossing Boulevard, Suite 101
Bridgewater, NJ 08807
Mobile: (401) 406-0358
convatec.com
Communication Type:
Discontinuation Notice
Vendor #10695
-
08/1/2024
Title: Hollister V#10901 - Ostomy Discontinuation Update
Details:
UPDATE 2024 Hollister Forma Flex Discontinuation Notice
2024 Ostomy FormaFlex Discontinuation FAQs CHANNEL
FormaFlex Cross Reference Channel Cross Ref.
08-05-24 Hollister Product Discon. Item Usage
Concordance Team,
Please see the attached important update regarding the Hollister Ostomy product portfolio.
As always, please reach out to Bill Black with any further questions you may have.
Thanks,
Communication Type:
Discontinuation Notice
Vendor #10901
-
07/31/2024
Title: Health-O-Meter V#10075
Details:
Dedicated to bringing you great value-based scales engineered with a purpose, the Health o meter® Professional hospital line was developed to improve workflow while keeping patients and caregivers safe. The newest state-of-the-art products include a fold-up wheelchair scale, a heavy-duty highly-mobile platform scale, and a high-resolution neonatal tray scale, leading the industry in antimicrobial protection, connectivity, accuracy, reliability, and safety. Other Acute Care scales include a stainless steel wet diaper/lap sponge/organ scale with an easy-to-read backlight and a patient transfer scale that weighs immobile patients instantly, shortening door to needle time. See the attached flyer to learn more about Health o meter® Professional’s new Acute Care scale line.
For more information, please reach out to your local MTMC Representative or contact Brett Lucido, Director of National Accounts blucido@homscales.com, (724) 799-4233.
thanks,
Kelly Virzi
Sales and Marketing Specialist
Pelstar LLC/Health o meter® Professional Scales
9500 West 55th Street
McCook, IL 60525-7110 USA
Direct Phone: 708-377-0549
weigheasier®
Communication Type:
Product Flyer
Vendor#10075
-
07/31/2024
Title: Medline V#10605 - Manufacturer Backorders
Details:
07-31-2024 DYND70372 MBO Item Usage
Concordance Team,
Medline is currently experiencing some minor backorders on item DYND70372 - Tray, Skin Scrub, Wet, Standard. However Medline also offers a VERY similar item, DYND70660 - Tray, Skin Scrub, Premium, Wet, LF.
Medline is well stocked on DYND706610 in your customer's branch. You can cancel the order DYND70372 and place an order for the same quantity on DYND70660. Since Medline has plenty of stock that order will fill immediately.
These items are virtually identical. The only major difference is that DYND70372 comes with Vinyl Gloves, whereas DYND70660 comes with Aloetouch Vinyl Gloves. Please see the BOMs below for reference. Please let me know if you have any additional questions.
DYND70372: DYND70660: 6 small, winged sponges 6 small, winged sponges 3 sponge sticks 3 sponge sticks 4 oz. of PVP scrub 4 oz. of PVP scrub 3 oz. of PVP paint 3 oz. of PVP paint 2 absorbent towels 2 absorbent towels 2 blue blotting towels 2 blue blotting towels 2 cotton tip applicators 2 cotton tip applicators Pair of Vinyl Gloves Pair of Aloe Touch Vinyl Gloves CSR Wrap CSR Wrap 4 compartment tray 4 compartment tray 2 compartment removable double tray If you have any further questions, please reach out to Bill Black.
Regards
Communication Type:
Backorder Notification/Update/Reports
Vendor #10605
-
07/30/2024
Title: Healthsmart V#10803 - New Product Arrival
Details:
Concordance Team,
Please see new product arrivals from Healthsmart.
If these items are not on your site, Healthsmart can fix that! Please let them know which products your customers are interested in, and they will provide you with additional information.
With any further questions you may have, please reach out to Bill Black.
Thank you,
Communication Type:
New Product Launch
Vendor #10803
Communication Type:
Product Changes
Vendor #10803
-
07/30/2024
Title: Medegen V#10423
Details:
Discontinued Item- C#129892
Dear Valued Customer,
We will no longer be able to sell item#9258 effective immediately. We apologize for the inconvenience.
Sold-To-Name
Sales Ord
Pur. Ord.
Material
Doc.Date
Sched.Date
Order Qty
UOM
Shipping.Pt
Ship Status
CONCORDANCE HEALTHCARE SOLUTIONS
752330
3302706
9258
7/15/2024
10/24/2024
2
CS
4000
Obsolete Item
Replacement item -
Item P00-49725
Desc- Kit Chemo Spill NS 12/cs
Pricing – $ 478.62
All open purchase order lines for the product have been cancelled. Please see the respective purchase orders listed below:
Thank you for your continued patronage. We value this relationship and as always, we continue to strive to be your preferred and most reliable supply partner.
Communication Type:
Discontinuation Notice
Vendor #10423
-
07/30/2024
Title: Stryker V#10613
Details:
Effective January 1, 2025, the Mistral-Air portfolio, formerly supplied by Stryker, will now be managed by TSC Life in the United States and Canada. This strategic move allows us to provide high-quality and innovative temperature management solutions directly to our valued customers nationwide. As the innovator and manufacturer of the Mistral Air portfolio, TSC Life has always been dedicated to excellence and innovation in our products. With a more pronounced presence in North America, we can ensure our customers receive the best possible service and support.
Please click here for additional information. If you have any questions, please reach out to Bill Black. Thanks,
Communication Type:
Updated Supplier Information
Vendor #10613
Communication Type:
Product Changes
Vendor #10613
-
07/29/2024
Title: BD V#10125
Details:
Update on Bactec
The attached customer letters are for your reference and to keep you informed on what is being communicated to customers by our Sales team.
We also have a BACTEC website for customers to reference for updates and FAQ’s
Please reach out to Bill Black if you have any questions. Thanks,
Communication Type:
Product Update
Vendor #10125
Communication Type:
New Product Launch
Vendor #10125
-
07/29/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_26Jul2024
Nestle Weekly Inv. Update Item Cross Ref.
07-29-2024 Nestle Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 07-22-2024. The following changes are being made:
- Compleat Pediatric Peptide 1.0 250ml was added
- Compleat Pediatric 1.5 250 ml was added
- Compleat 1.5 250 ml was added
- Compleat 1.5 Original was added
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
07/26/2024
Title: Cardinal V#10801 - Product Availability Update
Details:
Codes Removed from Hold_Sharps
07-26-2024 Product Availability Update Item Usage
Concordance Team,
Please see the attached listing of the SKU's that were removed from Quality Hold.
If you have any further questions, please reach out to Bill Black.
Regards
Communication Type:
Product Inventory Update
Vendor #10801
Communication Type:
Product Update
Vendor #10801
-
07/26/2024
Title: Puritan Medical V#10230
Details:
Temporarily Unavailable Items
Please see items attached notice for items now listed as Temp Unavailable with Puritan Medical. Please click here for Usage. Please click here for Customer listing.
If you have any questions please reach out to Jeff Shook. Thanks,
we are currently facing challenges due to raw material shortages, staffing constraints, and an unexpected surge in demand for our diagnostic products. These factors have strained our production capacity, leading to extended backorders and delays in providing accurate lead times for many of our products.
To address these issues, we are:
- Expanding Our Team: Actively recruiting additional operators to enhance our production capabilities.
- Optimizing Production Facilities: Relocating several business lines to our Pittsfield facility, which offers greater capacity. Please note that due to regulatory requirements and necessary validations, this transition will take some time.
- Improving Packaging Efficiency: Implementing changes to our packaging processes to improve efficiencies and control costs.
We aim to provide updated lead times for all our products within the next two weeks.
We appreciate your patience and understanding as we work diligently to meet your needs.
Megan Templet
Director of Sales-Midwest
Puritan Medical Products Company
MOBILE: 603.660.1278 | OFFICE: 207.876.3311 EXT 3316 | DIRECT: 207.876.1716
31 School St. | P.O. Box 149 | Guilford, ME 04443 | USA
Communication Type:
Temp Unavailable
Vendor #10230
-
07/25/2024
Title: Ansell V#11003
Details:
Ansell Glove discontinuation
RE: ENCORE® RADIATION ATTENUATION – PHASE OUT
Communication Type:
Discontinuation Notice
Vendor #11003
-
07/25/2024
Title: SC Johnson V#12219
Details:
Sales Roster Map
Communication Type:
Sales Roster
Vendor #12219
-
07/25/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 07-22-2024. Also attached is a usage report for the Adult Institutional Items from the last 6 months.
Please note: There was no usage information for PSN Deprioritized items from the last 6 months.
Please see the attachments listed below to reference this notification
- Abbott Institutional Inventory Letter 7_25_24
- Customer File Concordance Cross Ref.
- 07-26-2024 Abbott Weekly Inv. Update Item Usage
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
07/24/2024
Title: BD V#10125
Details:
New Allocation- BD PushButton
Please see below for new allocations with BD. Usage file with Customer listing is also linked here. If you have any questions please reach out to Bill Black. Thanks,
Sku
Description
% allocation of historical
Reason for Manual Allocation
367352
WINGSET PBBCS 21X.75 12 PREATTACHED
90%
To provide fair allocation to the market
368656
WINGSET PBBCS 23X.75 12 PREATTACHED
80%
To provide fair allocation to the market
368659
WINGSET PBBCS 25X.75 12 PREATTACHED
90%
To provide fair allocation to the market
367363
WINGSET PBBCS UTW PP 25X.75 12 LUER
100%
To protect current users from Sub issues
367364
WINGSET PBBCS UTW PP 23X.75 12 LUER
100%
To protect current users from Sub issues
367365
WINGSET PBBCS UTW PP 21X.75 12 LUER
100%
To protect current users from Sub issues
368687
UT-PBBCS 25 G 12 PAH
100%
To protect current users from Sub issues
368688
UT-PBBCS 23 G 12 PAH
100%
To protect current users from Sub issues
368689
UT-PBBCS 21 G 12 PAH
100%
To protect current users from Sub issues
Communication Type:
Allocation Notification/Update
Vendor #10125
-
07/23/2024
Title: Midmark V#10987 - Steam Sterilizer Selling Guide
Details:
Concordance Team,
Midmark has provided product notification that includes a selling guide for their new generation of steam sterilizers and further product info. Please see the links attached for further information.
With any further questions you may have, please reach out to Bill Black.
Regards
Communication Type:
Product Flyer
Vendor #10987
-
07/23/2024
Title: Mead Johnson V#10502
Details:
Please see attached letter from our leadership regarding the tornado that Indiana. Unfortunately, I don’t have specifics on which of your DC’s will affected. I should know mare information as the week progresses. I will keep you posted.
Thank you,
Rick Suddendorf
Executive National Accounts Manager DME/Home Health, Distribution & GPO
D +1 651-208-7383
richard.suddendorf@reckitt.com
Communication Type:
Updated Supplier Information
Vendor #10502
-
07/23/2024
Title: Abbott V#10575 - Product Availability Update
Details:
07-23-2024 Product Availability Update Item Usage
Concordance Team,
Abbott Nutrition is providing an update that the following SKU is available to order:
MFG# VAI Item Item Description 64803 248678 Nepro Van 8oz ARC This item will have quantities reviewed to ensure equitable access for patients across all channels.
Please review the attached item usage file.
If you have any further questions, please reach out to Mary Reinhart.
Regards
Communication Type:
Product Inventory Update
Vendor #10575
-
07/23/2024
Title: Cardinal V#10801 - Product Discontinuation Notice
Details:
2024 Distributor Material Updates ADDENDUM 07.19.2024 ACUTE
Per the attached 2024 Distributor Material Updates ADDENDUM 07.19.2024 ACUTE, the following items have been discontinued with no suggested replacements. Each one has been placed into item inactivation; once inventory depletes they will inactivate. All other discontinued items in the attached were previously addressed or not set up in the system. The New SKUs will not be set up in the system unless we receive a customer request.
S#769657 Mfg #2419S2
S#163012 Mfg #441209
S#163015 Mfg #441210
S#167992 Mfg #441215
S#300479 Mfg #6642
S#844019 Mfg #6647-
S#417279 Mfg #6648
S#793984 Mfg #9640-
S#800987 Mfg #9642-
If you have any further questions, please reach out to Bill Black.
Thanks,
Communication Type:
Discontinuation Notice
Vendor #10801
-
07/22/2024
Title: Thomas Medical V#10463 - Product Flyer
Details:
Concordance Team,
Thomas Medical has provided us with their latest Newsletter. Updates are as follows:
- Product Information
- Thomas Medical HSG Catheters
- Miller Advanced HSG Catheter
- Flexible & Shapeable HSG Catheters
- Steen Open - Tip HSG Catheter
- Thomas Medical HSG Procedural Tray
- Prep in on sterile field
- Video to learn more about their Catheters
- Survey
- Thomas Medical HSG Catheters
Please see the link attached above for further detail and information.
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Flyer
Vendor #10463
- Product Information
-
07/22/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_19Jul2024
Nestle Weekly Inv. Update Item Cross Ref.
07-22-2024 Nestle Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 07-15-2024. The following changes are being made:
- Boost Plus Vanilla came back
- Boost GC Vanilla came back
- Fibersource HN 1000 ml and 1500 ml came back
- Novasource Renal Vanilla came back
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
07/19/2024
Title: MOOG V#10244 - Infinity Pump Production Update
Details:
Infinity Pump Update 7-17-2024.pdf
Concordance Team,
MOOG Medical Devices Group has provided the attached PDF as a production update on their Infinity Pumps.
MOOG is currently shipping Infinity Pump and set orders every week. However, due to the volume of orders they are receiving, they are currently unable to fulfill all orders within the typical shipping timeframes their customers have grown accustomed to. This is due in part to the logistics of handling increased volumes of all their products as well as production working to keep pace with demand.
- Orders are estimated to be delayed 2 - 3 weeks
- Shipments are expected to return to normal by the end of August (subject to change based on order volumes received)
- It is recommended to place orders well in advance of needed shipment dates.
More updates to come. Please see the attachments above for further details.
With any questions you may have, please reach out to Bill Black.
Kindest Regards
Communication Type:
Product Update
Vendor #10244
-
07/19/2024
Title: Healthmark V#11133
Details:
Discontinued Items
Please see discontinued items and replacements for Healthmark
Communication Type:
Discontinuation Notice
Vendor #11133
-
07/18/2024
Title: Aspen Surgical V#10234 - Product Changes
Details:
07-18-2024 Product Change Item Usage
Concordance Team,
Aspen Surgical has provided a product change notice (Above) on the future color change to MFG#15211(CHS#129854) & MFG#15212(CHS#104794). This change is purely aesthetic and does not affect form, fit or function.
Please see the attachments above for further information.
With any further questions you may have, please reach out to Bill Black.
Regards
Communication Type:
Product Changes
Vendor #10234
-
07/18/2024
Title: GOJO V#10354 - New Sales Flyers/Brochures
Details:
PURELL Recommeded Formulary - Physician Market
PURELL Recommended Formulary – Ambulatory Surgery Center Market
PURELL Recommended Formulary – Dental Market (1)
PURELL Recommended Formulary - LTC Market
Concordance Team,
GOJO has provided us with a plethora of new sales flyers/brochures. Please see the attachments above for further information.
With any further questions you may have, please reach out to Bill Black.
Regards,
Communication Type:
Product Flyer
Vendor #10354
-
07/18/2024
Title: PDI V#10497 - Sales Team Map
Details:
Concordance Team,
PDI has provided Concordance with their most updated Sales Team Map. To locate your PDI Rep online, please visit: www.pdihc.com/rep
With any further questions you may have, please reach out to Bill Black.
Regards,
Communication Type:
Sales Roster
Vendor #10497
-
07/18/2024
Title: Abbott V#10575 - Weekly Inventory Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 07-08-2024. Also attached is a usage report for the Adult Institutional Items from the last 6 months.
Please note: There was no usage information for PSN Deprioritized items from the last 6 months.
Please see the attachments listed below to reference this notification
- Abbott Institutional Inventory Letter 7_18_24
- Customer File 7-18-24 Concordance Cross Ref.
- 07-18-2024 Abbott Weekly Inv. Update Item Usage
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
07/17/2024
Title: Baxter V#10797
Details:
Allocation and customer usage
Please see the attached letter regarding an allocation on Vial-Mate Adapters that is being implemented this evening. I will provide the allocation quantities as soon as they become available. Please let me know if you have any questions.
Regards,
Scott
Scott Evangelista
Associate Director, Trade Relations
Baxter Healthcare
847.274.4590
Communication Type:
Allocation Notification/Update
Vendor #10797
-
07/17/2024
Title: Kate Farms V#12409
Details:
New Product Launch
As noted, here are the 2 new SKUs we just launched. These are available to order right away if customers are asking for them. As mentioned on the phone, these products are used with those hospitals that have protocols for using pumps for enteral feeding.
Let me know if you have any questions or need more information.
Thank you for your continued support and partnership.
Greg Phipps
National Account Director, Sales Distribution
904-401-3080
katefarms.com
katefarmsmedical.comCommunication Type:
New Product Launch
Vendor #12409
-
07/17/2024
Title: Healthmark V#11133
Details:
New Product
Please see the attached product release memo for our newest line of cleaning chemistry products, XEN.
Thank you for your continued partnership!
Noah Cherry
Distributor Lead
Customer Service
(800) 521–6224 ext. 6787
Communication Type:
New Product Launch
Vendor #11133
-
07/17/2024
Title: Medical Developements Inter V#13587
Details:
have attached a one-page sheet with the order numbers included.
Can you please share with your team?
Also, if anyone would like samples, please send them my way.
Can you think of anything else to help the team?
Is there anyone we can meet with in person to help uncover more targeting etc?
The Global Head of Respiratory is touring the US In August the week of the 20th and we would enjoy being able to meet with anyone that may benefit the partnership.
Thank you,
Stephanie Hall
Communication Type:
Product SKU List
Vendor #13587
-
07/17/2024
Title: Medical Developments INTER V#13587
Details:
Product Flyer
This morning I received updated product sheets and leave behind materials
Please let me know if we can update them with any Concordance specific information.
Thank you!
Steph Hall
Communication Type:
Product Flyer
Vendor # 13587
-
07/17/2024
Title: Cardinal V#10801 - Product Discontinuations
Details:
2024 Distributor Material Updates ADDENDUM 07.05.2024 ACUTE
These items on the attachment have all been discontinued and inactivated.
S#227207 Mfg#2014- disc by mfg-no replacement
S#846733 Mfg#7006IC disc by mfg-no replacement
S#318744 Mfg#C-SUS66ZS disc by mfg-no replacement
S#299298 Mfg#M7170-1A disc by mfg-no replacement
Please see the attachment above for further information.
With any further questions, please reach out to Mary Reinhart.
Regards
Communication Type:
Discontinuation Notice
Vendor #10801
-
07/17/2024
Title: Lakeside Manufacturing V#10591 - Sales Rep Roster & Product Brochure
Details:
Lakeside Manufacturing Medical Brochure 2024
Concordance Team,
Lakeside Manufacturing has provided a brochure with medical products and their updated sales rep rosters.Please see the attachments above for further information.
With any further questions you may have, please reach out to Jeff Shook.
RegardsCommunication Type:
Product Flyer
Vendor #10591
Communication Type:
Sales Roster
Vendor #10591
-
07/17/2024
Title: Ansell V#11003
Details:
Ansell Sales Roster, Territory Map
Please see latest sales roster/territory map from Ansell
Communication Type:
Sales Roster
Vendor #11003
-
07/16/2024
Title: Nestle V#10183 - Weekly Nutrition Inv. Update
Details:
NHSc Weekly Inventory Letter_01Dec2023
12-04-2023 Weekly Inv. Update 2 Item Usage
Due to increased demand associated with formula shortages and supply chain challenges in the enteral space, Nestle
regrets to inform you that they may be in intermittent supply or potentially out of stock on the products listed below.Nestle is proactively working to minimize any disruptions and provide as much advance notice as possible regarding future
product availability.Please work with the patients’ healthcare providers to determine the best alternative product and transition plan.
For additional product information visit the Nestlé Medical Hub at www.nestlemedicalhub.com.- If you order through a Distributor
- Please contact your distributor to understand current inventory levels of impacted products.
- Please proactively communicate your anticipated substitute product choice and case usage to your distributor.
- Ask that additional volume be brought in to service the need short-term.
- Intermittent, as indicated on the “Estimated Recovery Date” or “Improved Availability” date, could result in orders being cut or limited supply through your preferred distributor.
- If you are a Distributor and/or order Direct from Nestlé
- Please check your order notifications for the most up to date information. You may call Customer Service with any questions at 877-463-7853 or email them at nhncustomerservice@us.nestle.com. Reference the PO number with the backordered line item.
- The notifications will provide an email confirming order creation, cut & shipping notifications.
- Customer manual POs can be submitted directly to:
- US: Nestle Solon BSCNHSc Esker Manual POs NestleSolonBSCNHScEskerManualPOs@us.nestle.com
- Please check your order notifications for the most up to date information. You may call Customer Service with any questions at 877-463-7853 or email them at nhncustomerservice@us.nestle.com. Reference the PO number with the backordered line item.
If you have any further questions, please reach out to Mary Reinhart.
Thanks,
Communication Type:
Product Inventory Update
Vendor #10183
- If you order through a Distributor
-
07/16/2024
Title: Midmark V#10987 - Product Update/Info.
Details:
BROCHURE - Midmark Instrument Processing 05.2024
BROCHURE - Midmark Sterilizers 05.2024
Concordance Team,
Midmark had provided us further information on the new Midmark Steam Sterilizers. The attached is good information received from a local rep and has all relevant messaging.
Below are all the skus that have been set up:
MFG Item No.
CHS#
Item Description
M9-050
401704
Midmark M9 Steam Sterilizer (115V) Medical/Dental
M11-050
401705
Midmark M11 Steam Sterilizer (115V) Medical/Dental
M11-051
401708
Midmark M11 Steam Sterilizer (230V)
9A694001
401712
Autofill System 115V
9A694002
401716
Autofill System 230V
9A703001
401722
Vertical Cassette Rack , M11-05X
9A698001
401725
USB Kit
9A586007
401726
VistaCool Adapter
With any further questions you may have, please reach out to Bill Black.
Regards
Communication Type:
Product Flyer
Vendor #10987
Communication Type:
Product SKU List
Vendor #10987
-
07/15/2024
Title: Baxter V#10797 - National Programs
Details:
Exam Tools Trade-Up April-December 2024 Promo Flyer-HR
Heart Health July-September National Promotion- LR
Q Stress April-September 2024 National Promotion-LR (1)
Spot Vision Screener Trade Up 2024 Promo Flyer
Concordance Team,
Please review the attachments for the national programs for Q3 2024 that Baxter has provided.
With any further questions you may have, please reach out to Bill Black.
Regards
Communication Type:
Updated Supplier Information
Vendor #10797
-
07/12/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_12Jul2024
Weekly Inv. Update Item Cross Ref.
07-17-2024 Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 006-17-2024. The following changes are being made:
- Diabetisource AC in the 1500 ml, 1000 ml and the 250 ml came back into stock
- Boost Plus Vanilla came back into stock.
- Novasource Renal Vanilla came back into stock.
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
07/12/2024
Title: ICU Medical V#10917 - Product Discontinuation Notice
Details:
Concordance Product Discontinuation 14248-28
Product Discontinuation Item Usage
Concordance Team,
ICU Medical has announced they are discontinuing MFG#14248-28 (CHS#183481) Primary PLUM Set Polyethylene Lined Tubing, Secure Lock, 107". ICU Medical is recommending their functionally similar product, MFG#15248-28 (CHS#401418) Primary PLUM Set CLAVE Secondary Port, Polyethylene Lined Tubing, Secure Lock, 107", as the replacement product. The replacement product, 15248-28, is available for immediate ordering from ICU Medical.
Please see the attachments above for further information.
With any further questions you may have, please reach out to Bill Black.
Regards,
Communication Type:
Discontinuation Notice
Vendor #10917
-
07/11/2024
Title: Cardinal V#10801 - Monoject Needles & Syringe Portfolio Availability Update
Details:
CPG Syringe Supply Health Customer Letter 07-03-2024
CPG Syringe Supply Health Excel 7.3.24
Concordance Team,
Cardinal has provided a customer letter updating us on the supply health of Cardinal Health Monoject Needle and Syringes.
- Cardinal is expanding upon their U.S. syringe production and actively evaluating alternate manufacturing options outside of China.
- Cardinal will provide updates as they have more information to share on overall plastic syringe supply.
- The Monoject Needles & Syringe portfolio will remain on allocation to protect overall supply health.
If you have any further questions, please reach out to Bill Black.
Regards,
Communication Type:
Allocation Notification/Update
Vendor #10801
-
07/11/2024
Title: Aspen Surgical V#10234 - MFG Location Change & Product Packaging Enhancements
Details:
JULY 2024 Manufacturing Update of ALC.docx
07-11-2024 Aspen Surgical Packaging Changes Item Usage
Concordance Team,
Aspen Surgical has provided the attached letter updating Concordance on the Manufacturing location change and packaging enhancements on their item #LT-ALC-01NS. Please see the Manufacturer Update attached for further information.
If you have any further questions, please reach out to Bill Black.
Regards
Communication Type:
Product Changes
Vendor #10234
Communication Type:
Product Update
Vendor #10234
-
07/11/2024
Title: Molnlycke V#10008
Details:
new Product launch
Dear Valued Distributor,
Mölnlycke Health Care, manufacturer of HibiClens® Antiseptic Skin Cleanser, is pleased to introduce a new line extension to our Hibi Universal Bathing System (HUBS)! Our new 5-count pack provides your hospital customers with a new option to meet specific patient bathing needs.
The Hibi Universal Bathing System 5-pack offers:
Flexibility:
- Our traditional HUBS pack included 10 dry, disposable washcloths in packaging that acts as a single-use basin. Our new 5 count pack provides for smaller patient populations, such as pediatrics, and additional uses where 10 cloths are not needed.
Improved sustainability:
- With half as many cloths as our traditional packaging, the new product avoids waste where fewer cloths are needed to support patient hygiene best practice.
An affordable entry point:
- Hospitals work hard to run sustainable businesses; our new line extension provides a lower price point than the 10-count pack to begin implementing bathing best practice.
All the benefits you expect from HUBS:
- Eliminate the infection risk associated with reusable basins, reduce reliance on laundered wash cloths, and experience the benefits of the nurse-aided bath. Our bathing system was designed to meet the in-use needs of nurses and the high demands of modern Infection Prevention practices.
Please contact your local representative or call the Molnlycke Customer Service Center at 1-800-843-8497 for more information. Thank you for your support and we look forward to continuing to help meet your infection prevention and patient hygiene needs.
Sincerely,
John and Bob
Bob Perry
Director, US Channel Management
Mölnlycke Health Care
Tel 949-306-0709
Web: www.molnlycke.com
Communication Type:
New Product Launch
V#10008
-
07/11/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 07-08-2024. Also attached is a usage report for the Adult Institutional Items from the last 6 months.
Please note: There was no usage information for PSN Deprioritized items from the last 6 months.
Please see the attachments listed below to reference this notification
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
07/9/2024
Title: Cardinal V#10801 - Allocation Update
Details:
CPG Syringe Supply Health Customer Letter 07-03-2024
Concordance Team,
Cardinal has provided a customer letter updating us on the supply health of Cardinal Health Monoject Needle and Syringes.
- Cardinal is expanding upon their U.S. syringe production and actively evaluating alternate manufacturing options outside of China.
- Cardinal will provide updates as they have more information to share on overall plastic syringe supply.
- The Monoject Needles & Syringe portfolio will remain on allocation to protect overall supply health.
If you have any further questions, please reach out to Bill Black.
Regards,
Communication Type:
Allocation Notification/Update
Vendor #10801
-
07/9/2024
Title: Midmark V#10987 - Product Info/Selling Guide
Details:
Concordance Team,
Midmark has provided a selling guide and product information for their next generation of steam sterilizers.Please see the attachments for further information.
If you have any further questions, please reach out to Bill Black.
Regards.
Communication Type:
New Product Launch
Vendor #10987
Communication Type:
Product Update
Vendor #10987
-
07/9/2024
Title: Thomas Medical V#10463 - News Letter
Details:
Concordance Team,
Thomas medical has provided us with their most recent newsletter. This newsletter covers the following topics:
1. Featured product for infertility care
2. Video on Thomas Medical Shapeable IUI
3. Recent Conferences
Please see the link attached above for further information on their Home Page, Product Catalog, Blog and Contact Page.
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Updated Supplier Information
Vendor #10463
-
07/9/2024
Title: Ansell V#11003 - Product Availability Update/ Discontinuation
Details:
Ansell Gammex PI Plus Glove-In-Glove Update Communication Letter July 2024
Concordance Team,
Ansell has sent out a reminder that the new Gammex PI Plus Glove-in-Glove system is now available. This updated configuration is an upgrade from the previous Gammex PI Glove-in-Glove system. The new upgraded system includes an unparalleled comfort and fit, as well as, Ansell's proprietary PI Kare Skin-Friendly, Non-Sensitizing PI Technology to address ongoing Type IV chemical allergy concerns, and a textured finish for exceptional instrument handling and grip.
The discontinued version of Gammex PI Glove-in-Glove system is no longer available for order placement as all stock has been depleted in Ansell warehouses. Please see the communication attached for new ordering information to update your systems accordingly. All future orders should utilize the new Gammex Pi Plus Glove-in-Glove system items as noted in the communication attached.
Please note, the only item from the Gammex PI Glove-in-Glove System we carry is the size 9 (MFG#340084090) CHS#346112. We do not have any usage on this item from the last 6months.
If you have any further questions, please see the attachments above and/or reach out to Mary Reinhart.
Regards
Communication Type:
Product Update
Vendor #11003
Communication Type:
Discontinuation Notice
Vendor #11003
-
07/5/2024
Title: Abbott Vendor#10575 - Product Availability Update 2
Details:
Concordance Team,
Abbott Nutrition has provided an update that the following SKUs are available to order. These items will have quantities reviewed to ensure equitable access for patients across all channels. You may still see these items on the weekly inventory notifications. They are just communicating updates on availability.
Please note: There has been no usage on this product in the last 6months.
MFG# VAI Item Item Description 62675 197950 Glucerna 1.2 Cal 1.5L RTH
With any further questions you may have, please reach out to Mary Reinhart.
Thanks,
Communication Type:
Product Inventory Update
Vendor #10575
-
07/5/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 07-01-2024. Also attached is a usage report for the Adult Institutional Items from the last 6 months.
Please note: There was no usage information for PSN Deprioritized items from the last 6 months.
Please see the attachments listed below to reference this notification
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
07/3/2024
Title: Tidi V#10260
Details:
MMCAP FLYER/Product detail
The attached e-mail includes both flyer sheets and links to our website. Also, there are 12 sku’s; 3 PenBlade and 9 Eye/Face Protection.
Any questions, please let me know.
Thank you!
Denise Sitzberger
National Account ManagerCell: 616.450.5614
dsitzberger@tidiproducts.com | www.tidiproducts.com
Communication Type:
Product Flyer
V#10260
Communication Type:
Product SKU List
V#10260
-
07/3/2024
Title: Invacare V#10748
Details:
Adam Sitzmann
National Channel Manager
Invacare North America
Mobile: 813-951-2834
Email: asitzmann@invacare.com
One Invacare Way
Elyria, OH 44035
With this new Essentials™ Mattress, we prioritized both quality and safety. The CertiPUR-US® certification ensures our high-quality mattress foam is made without harmful chemicals and boasts low VOC emissions for better indoor air quality.
Ready to transform patient care with our cost-effective solution? Please don't hesitate to reach out to me to ask questions, or ask for a quote!
Communication Type:
New Product Launch
Vendor #10748
-
07/2/2024
Title: CryoConcepts V#12777
Details:
Product Brochure
Attached is a price increase notification for the CryoLab Medical device and Brochure This is our first price increase on any U.S. medical product in nearly 7 years. This particular product is due for an increase based on the our rising materials costs but also because it has been well accepted in the market with average selling price increasing organically through distribution.
Please let me know if you have any questions or if I can provide anything further.
Best regards,
Ken
Ken Stoner
Vice President
North American Sales
O: 484-894-2786
M: 864-915-0096
Communication Type:
Product Flyer
Vendor #12777
-
07/2/2024
Title: Conmed V#10716
Details:
packaging changes
Good morning Team,
CONMED has been implementing several Green Initiatives, the removal of the inner boxes within the custom pack ECG Electrode cases is the latest Green Initiative. Moving forward, all custom pack ECG Electrodes will be loosely placed inside the case instead of packed within inner boxes. We do apologize for any inconvenience this may have caused. We believe that reducing our carbon footprint is not only beneficial to CONMED but also aligns with the values we share with our customers. If you have any questions or need any additional information, please let me know. Thank you and hope you have a great day.
James Topelski
Director of Sales, AET Patient Monitoring
11311 Concept Blvd, Largo, FL 33773
Mobile: (407) 923-1700
Web: www.CONMED.com
Email: jamestopelski@CONMED.com
Communication Type:
Product Update
V#10716
-
07/2/2024
Title: Perrigo Direct V#13554
Details:
We conducted research with 600 moms of babies 0-12 months in age. One of the key insights was how much they need the support and guidance of their pediatric healthcare team!
You are a mom's most trusted resource when there are concerns about infant feeding. How much do you know about them?Communication Type:
Product Update
V#13554
-
07/2/2024
Title: Jant V#10420
Details:
End User Promo
Jant V#10420, is offering an Q3 End-User Promo on items in the attached file. Also attached are the corresponding CHS#’s. I am having IM set up the one item on the list that we do not have active at this time, which is the Acutest Reader, PRM 395. Promo offerings will be determined by sales tracings and sent directly to the customer at the end of the program. Please direct any questions to Tiffany or myself and we will be glad to help in any way possible. All this information will also be published in HUBSPOT for future reference and need.
Tiffany Mathias
818.203.2410
Regional Sales ManagerAs Always, thank you for all your efforts, it is truly appreciated.
Have a safe and Happy 4th of July!
Best Regards,
--------------------------------------------------
Mary Reinhart | Supplier Relations, Manager
Concordance Healthcare Solutions
85 Shaffer Park Drive, Tiffin, OH 44883
419-455-2162 | mreinhart@concordancehs.comCommunication Type:
End User Promo
V# 10420
-
07/2/2024
Title: BD V#10125 - UPDATE: Glove Supply Disruption in Foley Catheter and Intermittent Catheter Trays
Details:
NPM-4103 Acute Care Customer Letter - Glove update 6-26-24
Concordance Team,
Please see the update from BD Regarding the Glove Supply Disruption in Foley Catheter and Intermittent Catheter Trays below:
Thank you for your patience while we worked to address the supply disruption affecting the sterile gloves in our Foley catheter and Intermittent catheter trays. We are pleased to share that we have successfully identified a solution for this challenge and are in the process of integrating the new glove supply into production.
Key Updates:
Sterile Gloves Availability: Customers can expect to begin seeing sterile gloves in our Foley catheter and Intermittent catheter trays in August. Exact timing will be dependent on inventory levels at each account or distribution/self-distribution warehouse.Yellow Sticker Identification: To facilitate easy differentiation, trays containing sterile gloves will not bear the yellow sticker on the outer label. This visual cue will help you quickly identify which trays include gloves and which do not.
If you have any further questions, please reach out to Bill Black.
Thank you,
Communication Type:
Product Update
Vendor #10125
-
07/2/2024
Title: Rockwell V#10934 - CENTRISOL and RENASOL Discontinuation
Details:
Centrisol and Renasol Concentrate Formulations Catalog
Rockwell Medical Concentrates Product Catalog
Concordance Team,
Please see the product discontinuation notice below and the associated attachments.
Rockwell Medical will supply you with the following recommended substitute concentrates from our RenalPure® and SteriLyte® product lines:
-
CENTRISOL and RENASOL liquid acid gallons will be substituted with RenalPure liquid acid gallons.
-
CENTRISOL and RENASOL liquid bicarbonate gallons will be substituted with Sterilyte liquid bicarbonate gallons.
Product Substitute Recommendations: Enclosed you will find a comprehensive CENTRISOL and RENASOL product portfolio list along with our recommended RenalPure and SteriLyte product substitutions and product formula details. The products listed in the conversion tables are suggested substitutes only. Please exercise caution, refer to a physician or clinician to confirm appropriate use before administering to a patient, and refer to machine manufacturer for assistance with calibration changes.
-
Rockwell Medical CENTRISOL and RENASOL Product Conversions (Excel)
-
Rockwell Medical CENTRISOL and RENASOL Product Conversions (PDF)
Pricing: Please note that there will be no change to your product pricing at this time. (i.e. you should expect to pay the same price for a RenalPure liquid acid case as a CENTRISOL liquid acid case or RENASOL liquid acid case).
Ordering: Orders should be emailed to our Rockwell Medical Customer Care team at custserv@rockwellmed.com.
Additional information will be provided in the coming weeks. Please contact sales@rockwellmed.com with any questions you have about this transition and refer to RockwellMedical's Product Catalog for additional information on RenalPure and SteriLyte or any other products in our portfolio. We look forward to continuing our business with you.
Sincerely,
Tim Chole
Chief Commercial Officer
Rockwell Medical, Inc.
With any further questions, please reach out to Bill Black.
Regards
Communication Type:
Discontinuation Notice
Vendor #10934
-
-
07/2/2024
Title: Solventum/3M V#10296 - Littmann Stethoscopes August 2024 MAP
Details:
Littmann MAP Policy_eff. March 1, 2023
07-05-2024 Littman MAP Item Usage
Concordance Team,
Solventum/PKA 3M is pleased to announce MAP holiday August 1-31, 2024 exclusively for 3M Littmann CORE Stethoscope, 8480&8483.
Please note the MAP Policy remains in effect during this time, and the change is the lowered MAP only on 3M Littmann CORE Stethoscope, 8480, Black, Eko. All other MAP products are not on holiday.
Please see MAP highlighted in the following table:
Material Cat# CHS# Billing Unit 2024 August MAP 3M™ Littmann® CORE Stethoscope, 8480, Black 8480 332854 Each $299.00 3M™ Littmann® CORE Stethoscope, 8570, HP Rainbow 8570 357859 Each $349.00 3M™ Littmann® CORE Stethoscope, 8870, HP Copper 8870 - Each $349.00 3M™ Littmann® CORE Stethoscope, 8890, Mirror 8890 - Each $349.00 3M™ Littmann® CORE Stethoscope, 8483, Black, Eko 8483 - Each $299.00 3M™ Littmann® CORE Stethoscope, 8574, HP Rainbow, Eko 8574 - Each $349.00 3M™ Littmann® CORE Stethoscope, 8838, HP Copper, Eko 8838 - Each $349.00 3M™ Littmann® CORE Stethoscope, 8839, Mirror, Eko 8839 - Each $349.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Black Tube, 22 inch, 6151 6151 235533 Each $199.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Black Tube, 27 inch, 6152 6152 235537 Each $199.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Navy Blue Tube, 27 inch, 6154 6154 235540 Each $199.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Hunter Green Tube, 27 inch, 6155 6155 235541 Each $199.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Plum Tube, 27 inch, 6156 6156 235542 Each $199.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Raspberry Tube, 27 inch, 6158 6158 235544 Each $199.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Rose Pink Tube, 27 inch, 6159 6159 242502 Each $199.00 3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Burgundy Tube, 27 inch, 6184 6184 - Each $199.00 A copy of the current MAP Policy and item usage is attached for further reference.
With any further questions, please reach out to Bill Black.
Regards,
Communication Type:
End User Promo
Vendor #10296
-
07/1/2024
Title: Abbott V#10575 - Product Availability Update
Details:
Customer Letter Tetra Availability
Nepro Distributor Order Sheets_All Other_Final 6.25
Availability Update Item Cros Ref.
07-03-2024 Availability Update Item Usage
Concordance Team,
Abbott is happy to share that effective immediately, Ensure Original Vanilla 8oz ARC and Ensure Plus Vanilla 8oz ARC are available to order. Abbott is also requesting that we transition these items back to the 8oz ARC table in the Availability Update Item Cross Ref. Attachment above.
In addition, Nepro Vanilla 8oz ARC will be available to order beginning August 2024. Abbott is also requesting that we transition these items back to the 8oz ARC table in the Availability Update Item Cross Ref. Attachment above.
With any further questions you may have, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
Communication Type:
Product Update
Vendor #10575
-
06/28/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 06-21-2024. Also attached is a usage report for the Adult Institutional Items from the last 6 months.
Please see the attachments listed below to reference this notification
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
06/28/2024
Title: Nestle V#10183 - Nutramen 1.5 & Peptamend Intense VHP Update
Details:
Concordance Backorder Report for Nutren 1.5 & Peptamend Intense VHP
Nutramen 1.5 & Peptamend Intense VPH Item Usage
Concordance Team,
Nestle has two allocations set aside for Concordance, one for Nutren 1.5 1000 ml and another for Peptamen Intense VHP 1000 ml, see below. Please let me know which backorders to fulfill on the attached backorder report.
NUTREN® 1.5, SpikeRight® PLUS 6 x 1000 mL UltraPak® bags-25 Cases
9871626354
PEPTAMEN® INTENSE VHP, SpikeRight® PLUS 6 x 1000 mL UltraPak® bags-69 Cases
4390049322
Please refer to the attachments above, for further information.
With any further information you may have, please reach out to Mary Reinhart.
Regards,
Communication Type:
Allocation Notification/Update
Vendor #10183
-
06/28/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_28Jun2024
Weekly Inv. Update Item Cross Ref.
06-28-2024 Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 006-17-2024. The following changes are being made:
- Boost GC Vanilla was added as a low product
- Boost Kids Essential Chocolate was added as a low product
- Boost Kids Essential Strawberry was added as a low product
- Fibersource HN 1000 ml was added as a low product
- Fibersource HN 1500 ml was added as a low product
- Peptamen Jr PHGG 250 ml was added as a low product
- Novasource Renal 1000 ml was removed
- Peptamen 1.5 1000 ml was removed
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
06/26/2024
Title: ICU V#10917 - Product Discontinuation Notice
Details:
ICU 14248-28 Discontinued Product Notification JCG 6.26.24.pdf
COncordance Product Discontinuation 14248-28
Concordance Team,
Please see the product discontinuatino notice from ICU Medical. Attached is an excel file of our open orders that will need to be cancelled as ICU will not be fulfilling these orders. Please see the information from the supplier below and as attached.
Dear Valued Trading Partner,
ICU Medical has exhausted our on-hand inventory of product number 14248-28, Primary PLUMTM Set Polyethylene
Lined Tubing, Secure Lock, 107 “. We are discontinuing sales of 14248-28 effective immediately. All unfulfilled purchase
orders for 14248-28 will be cancelled. Future Orders for the discontinued product will be Rejected. Returns of the
discontinued product will not be accepted for credit per ICU Medical’s Return Good policy.
Discontinued Product
ICU Medical Item #
Description
14248-28
Primary PLUMTM Set Polyethylene Lined Tubing, Secure Lock, 107 “
ICU Medical is recommending our functionally similar product, 15248-28, Primary PLUMTM Set CLAVE Secondary Port,
Polyethylene Lined Tubing, Secure Lock, 107“, as the replacement product. The replacement product, 15248-28,
is available for immediate ordering from ICU Medical.
Replacement Product
Item #
Product Description
GTIN
PUOM
CASE Size
Acquisition Case Price
15248-28
Primary PLUMTM Set CLAVE Secondary Port, Polyethylene Lined Tubing, Secure Lock, 107“
Case - 10887709102344
Each - 00887709102347
CS
50
$847.50
With any further questions you may have, please reach out to Bill Black.
Regards,
Communication Type:
Discontinuation Notice
Vendor #10917
-
06/26/2024
Title: MHC Medical V#12615 - Product Update
Details:
2f396513-52ca-42e5-910b-c0d4aa886d7a
e009aeae-5a50-47f7-8b4d-2a189cdea0d8
Concordance Team,
MHC has received some calls from the other big companies looking from the 1ML barrel without needle, 802010(TB) & 802815(INS). Any recalls do not affect MHC.
MHC is also updating their BPMs. Same item number, just upgraded. This item 860213 for $19.99 in attachment.
Please see the attachments for further information.
With any further questions you may have, please reach out to Bill Black.
Regards,
Communication Type:
Product Update
Vendor #12615
-
06/26/2024
Title: Medegen V# 10423 - Product Discontinuation Notice
Details:
Concordance Team,
Please see below for important information regarding the product discontinuation notice on Medegen's Sterile Bowls, CHS#997452 and CHS#561118.
Due to ongoing challenges with continued production of our sterile bowls, listed below, we will no longer be able to offer these products. We will continue to explore the possibility of supplying these products again in the future.
Below are additional details regarding inventory position and plans for discontinuation:
01232 Bowl: 32oz Peelpouch 50/Cs : We have extremely limited inventory remaining and will notify separately regarding order lines to be cancelled.
01216 Bowl: 16oz Peelpouch 75/Cs 16/plt: We still have inventory of the 16 oz bowl and expect to have supply for approximately 45 days; we will not accept orders that are above historical ordering patterns for any customer. Once supply is depleted, we will notify of final discontinuation status and let you know about any order lines that have to be cancelled at that time.
We can recommend the following non-sterile bowls as possible other options, please contact either your sales representative or Customer Service for further details:
16oz
04216 Bowl 16oz Turquoise 100/cs
PO1629-500 Bowl: 16oz Blue RS NS 500/Cs
32oz
02433 Bowl 32oz White NS 250/cs 24/plt
07332 Bowl 32oz Turquoise NS Bulk NS 100/cs 72
PO1630-250 Bowl 32oz Blue RS NS 250/cs
We apologize for the inconvenience. Please contact us if you have further questions.
Sincerely,
Customer Service Team
Inteplast Group – Medegen Medical Products
360 Motor Parkway, Suite 800
Hauppauge, NY 11788If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Discontinuation Notice
Vendor #10423
-
06/24/2024
Title: B. Braun V# 10508 - Allocation Update
Details:
Concordance Team,
Please see the items listed below that have been removed from allocatino and a customer listing is attached of users that will need notified.
MFG#
CHS#
VA CHS#
R5000-01
726505
243612
R5001-01
640102
R5200-01
726497
243616
R5201-01
314559
R5310-01
142889
R5410-01
148412
R5510-01
606165
R6600-01
359118
R6601-01
606168
With any further questions you may have, please reach out to Mary Reinhart.
Regards,
Communication Type:
Allocation Notification/Update
Vendor #10508
-
06/24/2024
Title: Thomas Medical V#10463 - Product Survey
Details:
Concordance Team,
Please see the link included above to complete the survey requested by Thomas Medical.
Regards,Communication Type:
Product Survey
Vendor # 10463
-
06/24/2024
Title: Safetec V#10044 - OSHA Requirement
Details:
Concordance Team,
Did you know?
If you have a FIRST AID KIT you are required to have a SILL KIT in order to be OSHA compliant.
If you're selling First Aid products, you should also be selling spillkits.
Safetec offers a variety of spill kits for many typpes of workplace hazards, like their Universal Precaution Compliance Kit.
This kit meets the OSHA Standard 29 CFR 1910, 1030, which is all about protecting workers from the health risks associated with exposure to blood and other potentially infections materials.
Safetec offers the following types of kits and many more:
National Standard EZ - Cleans Kit
EZ - Cleans Plus Kit
EZ - Personal Protection Kit
Visit their ANSI compliance resource page for products, collateral, and additional information!
Visit their OSHA compliance resource page for products, collateral, and additional information!
With any further questions you may have, please reach out to Mary Reinhart.
Regards,Communication Type:
Product Flyer
Vendor #10044
-
06/24/2024
Title: Cardinal V#10801 - Kendall Medical and Surgical Tape Product Change
Details:
June 2024 Tapes Customer Letter
06-27-2024 Cardinal - Kedall Medical & Surgical Tape Item Usage
Concordance Team,
Cardinal has provided a customer letter regarding a change in their Medical and Surgical Tapes.
If you have any further questions, please reach out to Bill Black.
Regards,
Communication Type:
Product Changes
Vendor #10801
-
06/24/2024
Title: Cardinal V#10801 - Product Discontinuation Notice
Details:
Concordance Team,
This communication is to inform you of changes to the Cardinal Health Operating Room Accessories portfolio. At Cardinal Health, they continually evaluate their product portfolios to ensure that we offer clinically relevant products that provide the best value to our customers.
Please see the attachment for further information.
Regards,
Communication Type:
Discontinuation Notice
Vendor #10801
-
06/24/2024
Title: Midmark V#10987 - Steam Sterilizer Selling Guide
Details:
Concordance Team,
Midmark has provided the selling guide for the next generation of Midmark Steam Sterilizers. They are designed with intuitive instructions that can simplify compliance, cycle operation and performing and recording device care. This can help reduce transcription and documentation errors which could account for 56% of sterilization errors. Automated mechanical monitoring and chemical indicator reporting can save time, helping simplify staff training and giving them confidence to process instruments.
See how a new, intuitive user interface, streamlined compliance recordkeeping and a longer device life ca simplify workflow, safety and compliance
To view pricing please see the link

Please see the link provided for further information.
With any further questions, please refer to Bill Black.
Regards,
Communication Type:
Product Changes
Vendor #19887
-
06/21/2024
Title: Detecto V#10793 (NDC V#10030) - Waste Mates Update
Details:
Detecto Waste Mate Plastic Waste Receptacles Bulletin.pdf
Concordance Team,
MedPro is the Rep Firm !!
Great alternative to Rubbermaid.
No minimums, ships quickly and no drop ship fees.
Can be a profitable item to sell!
With any further questions, please reach out to Bill Black.
Regards
Communication Type:
Product Flyer
Vendor #10793/10030
Communication Type:
Product Update
Vendor #10793/10030
-
06/21/2024
Title: BD V#10128 - BD Bactec Supply Update
Details:
June 2024 - BD BACTEC Blood Culture Media Supply.pdf
BD Diagnostics 6mo Usage in Units.xlsx
Concordance Team,
Attached is letter from BD on Bactec Blood Culture items. They have shared with us today that we need to put all customers on a 50% allocation.
They are asking you all to reach out to your local BD rep to have a joint discussion with Customers.
I have attached a 6 month usage file of these items with your accounts that are effected.
Please let Bill Black know if you have any questions or issues reaching your local BD rep.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10128
-
06/21/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 06-21-2024. Also attached is a usage report for the Adult Institutional Items from the last 6 months.
- Please see the attachments listed below to reference this notification
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
06/21/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_21Jun2024
06-27-2024 Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 006-17-2024. The following changes are being made:
- Nutren Junior w/fiber came back into stock
- Boost Breeze Orange is out of stock
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
06/20/2024
Title: MicroCare V#10201
Details:
ProEZ 2 highlights
Hi Concordance Team,
ProEZ 2 is our product spotlight for June. ProEZ 2 is our dual enzyme (Protease & Amylase) detergent.
UOM – 4 Gallons/Case
Applications:
- Suitable for cleaning medical, dental and veterinary instruments, and devices
- Presoak and manual sink cleaning
- Ultrasonic instrument cleaning devices
- Manual endoscope cleaning and automated cleaning cycles of AERs
- Clean exterior of transducers and ultrasound wands
Features:
- True dual enzyme formula includes protease enzymes for most common protein soils and amylase enzymes for challenging gastrointestinal starch soils
- Specialty chemistry with enhanced cleaning for endoscopes and devices used for intestinal surgery
- Tested and validated for fast soil breakdown during soaking and sonication
- Non-corrosive with broad material compatibility including aluminum
- Neutral pH, enzymatic cleaner meets device manufacturer’s cleaning directions
- Chelating agents improve cleaning action in hard water
- Made in the USA
Please let me know if you have any questions or concerns. I am available for training & product education via phone or MS Teams.
Thanks,
Charlie Campbell
Key Account Manager
Microcare LLC | 6120 East 58th Avenue Commerce City, CO 80022
Email: CharlieCampbell@MicroCare.com| Mobile: 720.812.2432
www.Microcare.com/certol | www.ACIDMagic.com
Communication Type:
Product Flyer
Vendor #10201
-
06/19/2024
Title: Arkray V#10391
Details:
New Sales Roster
I would like to get it out to the ARKRAY Field Sales folks for more interaction especially with our PT Care conversion promotional sales push.
Also, please see attached for the ARKRAY most updated sales roster for your records.
Thanks.
Richard Slouffman
Vice PresidentProfessional Health Division
ARKRAY USA, Inc.
Your Diabetes Health Ally
CELL 513.607.6060
arkrayusa.com
To opt out of future email communications, please click here.
This electronic mail (including any attachments) may contain information that is privileged, confidential, and/or otherwise protected from disclosure to anyone other than its intended recipient(s). Any dissemination or use of this electronic email or its contents (including any attachments) by persons other than the intended recipient(s) is strictly prohibited. If you have received this message in error, please notify us immediately by reply email so that we may correct our internal records. Please then delete the original message (including any attachments) in its entirety. Thank you.
Communication Type:
Sales Roster
Vendor #10391
-
06/18/2024
Title: Smiths Medical V#10642
Details:
BLUselect Tracheostomy Tube Field Notification Recall
Dear Bill Black,
This is to inform you of a BLUselect Tracheostomy Tube Field Notification.Our partner Sedgwick will be sending an information package to any affected customers via US Mail with priority tracking. The notification details the issue, the affected items, and the required steps to perform.
For further inquiries, please contact Sedgwick by email or phone…
smithsmedical8551@sedgwick.com
1-888-628-0731 (M–F 8am–5pm ET)
Chris Jackson
Manager, Distribution.US
600 North Field Drive
Lake Forest, IL 60045
Phone: 206.457.7108
Email: Chris.Jackson1@ICUMed.com
Follow us on:
Facebook > Twitter > LinkedIn > YouTube > Google+ > Pinterest
Communication Type:
Recall Notification
Vendor #10642
-
06/18/2024
Title: PDI V#10497
Details:
PDI promo sales sheets
Hello everyone!
Please update existing PDI Sani-Hands literature with the attached:
- **NEW** PDI Sani-Hands sell sheet & promotion attached.
- Please distribute to your sales teams/members & update in vendor portal for PDI
- For PDI samples & pricing assistance, please contact your local PDI Rep
To locate your local PDI rep, visit: www.pdihc.com/rep
If you need additional information or assistance, please contact me directly. Thank you!
Michael S. Dalbom
Director, NATIONAL ACCOUNTS | NON-ACUTE400 Chestnut Ridge Road I Woodcliff Lake, NJ 07677
- 913.488.3715
Communication Type:
End User Promo
Vendor #10497
Communication Type:
Product Flyer
Vendor #10497
-
06/17/2024
Title: Cardinal V#10801 - Kendall Medical & Surgical Tapes Update
Details:
Final- CPG Tapes Customer Letter June 2024
Concordance Team,
To stabilize supply and mitigate backorders, Cardinal has qualified a new supplier for the Kendall™ Medical and Surgical tapes portfolio. While there are some changes to the look and feel of the product, the material meets or exceeds their product specifications. Please see the attached letter for additional details.
With any further questions you may have, please reach out to Bill Black.
Regards,
Communication Type:
Backorder Notification/Update/Reports
Vendor #10801
Communication Type:
Product Inventory Update
Vendor #10801
Communication Type:
Product Update
Vendor #10801
-
06/14/2024
Title: Nestle V#10183 - Impact Peptide 1.5 Ingredient changes
Details:
Nestle Health Science Distributor Communication June2024
Nestle Health Science Customer Communication June2024
Side by side Impact Peptide 1.5 vs Peptamen AF Peptamen 1.5 Peptamen Intense VHP.pdf
06-10-2024 Nestle Impact Peptide 1.5 Item Usage
Concordance Team,
Nestle has provided ingredient changes to their formulas on Impact Peptide 1.5 product line. The reason for the change is accessibility and the guaranteed capability to have raw ingredients for the manufacture of their portfolio. Please see the attachments to see exactly what is changing and for further information.
With any further questions, please reach out to Mary Reinhart.
Best Regards,
Communication Type:
Product Changes
Vendor #10183
-
06/14/2024
Title: Abbott V#10575 - Weekly Inv. Updated
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 06-03-2024. Also attached is a usage report for the Adult Institutional Items from the last 6 months. There was no usage information for the PSN Deprioritized items listed in the Inventory Letter.
-
- Please see the attachments listed below to reference this notification
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
-
06/14/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_14Jun2024
Nestle Weekly Inv. Update Item Cross Ref.
06-21-2024 Weekly Inv. Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 006-10-2024. The following changes are being made:
- Nutren 2.0 1000 ml was removed
- Boost Plus Vanilla was removed
- Fibersource 1000 ml and 1500 ml came back
- Peptamen AF 1000 ml came back
- Compleat Original 1000 ml came back
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
06/13/2024
Title: BD V#10128 - Supply Update
Details:
June 2024 - BD BACTEC Blood Culture Media Supply.pdf
06-13-2024 BACTEC Blood Culture Supply Media Item Usage
Concordance Team,
BD has informed we may experience intermittent delays in supply of some BD BACTEC Blood Culture Media supply over the coming months. The impacted product is as follows:
MFG# CHS# Product Name 442023 603972 BD BACTEC Plus Aerobic/F Culture Vials 442021 264799 BD BACTEC Lytic/10 Anaerobic/F Culture Vials 442020 252844 BD BACTEC Peds Plus/F Culture Vials 442022 363055 BD BACTEC Plus Anaerobic/F Culture Vials 442024 295513 BD BACTEC Standaard Anaerobic/F Culture Vials 442027 295515 BD BACTEC Standard/10 Aerobic/F Culture Vials 442794 #N/A BD BACTEC Myco/F Lytic Culture Vials With any further questions you may have, please reach out to Bill Black.
Regards,
Communication Type:
Product Inventory Update
Vendor #10128
-
06/13/2024
Title: Mar-Med V#11151
Details:
BEZ Labeling
Hi,
As a Balloon Extractor customer, please see the attached letter regarding new features included on our Balloon Extractor label. Let us know if you have any questions or if this information has been helpful.
Best,
Sara Werkema
Mar-Med
333 Fuller Ave N.E.
Grand Rapids, MI 49503
(616) 454-3000
Communication Type:
Label Changes
Vendor #11151
-
06/12/2024
Title: Midmark V#10987 - Extended Warranty Promotion
Details:
2024 Vital Signs Extended Warranty Promotion Details.pdf
Concordance Team,
Midmark has provided the attached to include further information on their new offer of a 4 year warranty. Please see the attachement for further information.
With any further questions, please reach out to Bill Black.
Regards,
Communication Type:
End User Promo
Vendor #10987
-
06/11/2024
Title: Midmark V#10987 - Steam Sterilizer Product
Details:
midmark-steam-sterilizers-ebook
Concordance Team,
Midmark has provided us with further information on how they are making things easier for nursing and hospital staff with the release of their "next generation of steam sterilizers".
The next generation of Midmark M9 and M11 Steam sterilizers are designed to help make instrument processing as easy and automated as possible. See why these devices are a smart investment in the attachment above.
If you have any further questions, please reach out to Bill Black.
Regards
Communication Type:
New Product Launch
Vendor #10987
Communication Type:
Product Update
Vendor #10987
-
06/7/2024
Title: Graham Field V#10228 - Supplier Partner Graham Field
Details:
Concordance Team,
Please review this months spotlight from supplier partner Graham Field V#10228.
Feel free to reach out to you rlocal rep or Grang Lange (Contact info below) with any questions or assistance needed on any sales per Bill Black.

Best regards,
Communication Type:
Product Update
Vendor #10228
-
06/7/2024
Title: Zoll V#10276 - Alternate Care Sales Roster
Details:
Alternate_Care_ Map CURRENT. pdf
Concordance Team,
Zoll Medical Corporation has provided their most current Alt. Care Site Sales Roster Map. Please see the attachment provided for further information.
Please reach out to Bill Black with any further questions.
Best Regards
Communication Type:
Sales Roster
Vendor #10276
-
06/7/2024
Title: Thomas Medical V#10463 - Smoke Evacuator System
Details:
Concordance Team,
Please see the information below regarding Thomas Medical V#10463.
With any further questions, please reach out to Mary Reinhart.
View this email in your browser 













Copyright © 2022 Thomas Medical, Inc., All rights reserved.
Subscribers receive promotional information from Thomas Medical, Inc.Regards
Communication Type:
Product Update
Vendor #10463
-
06/3/2024
Title: Cardinal V#10801 - Product Availability Update
Details:
ProductAvailabilityUpdateCAHBrand.pdf
Concordance Team,
Cardinal has provided a product availability update as of 05-31-2024. Please refer to the attachment above for further information.
Please reach out to Bill Black with any further questions you may have.
Regards,
Communication Type:
Product Inventory Update
Vendor #10801
-
06/3/2024
Title: Solventum V#10296 - Littmann Stethoscopes 2024May MAP
Details:
Littmann MAP Policy_eff. March 1, 2023
06-03-2024 Littmann MAP Notice Item Usage
Concordance Team,
This is a reminder that 2024 May nurse Appreciation Month MAP ended May 31, 2024.
2024 MAP in the following table will be effective on June 1st.
Material
Cat#
Billing Unit
2024 MAP
3M™ Littmann® CORE Stethoscope, 8480, Black
8480
Each
$329.00
3M™ Littmann® CORE Stethoscope, 8570, HP Rainbow
8570
Each
$349.00
3M™ Littmann® CORE Stethoscope, 8870, HP Copper
8870
Each
$349.00
3M™ Littmann® CORE Stethoscope, 8890, Mirror
8890
Each
$349.00
3M™ Littmann® CORE Stethoscope, 8483, Black, Eko
8483
Each
$329.00
3M™ Littmann® CORE Stethoscope, 8574, HP Rainbow, Eko
8574
Each
$349.00
3M™ Littmann® CORE Stethoscope, 8838, HP Copper, Eko
8838
Each
$349.00
3M™ Littmann® CORE Stethoscope, 8839, Mirror, Eko
8839
Each
$349.00
3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Black Tube, 22 inch, 6151
6151
Each
$199.00
3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Black Tube, 27 inch, 6152
6152
Each
$199.00
3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Navy Blue Tube, 27 inch, 6154
6154
Each
$199.00
3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Hunter Green Tube, 27 inch, 6155
6155
Each
$199.00
3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Plum Tube, 27 inch, 6156
6156
Each
$199.00
3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Raspberry Tube, 27 inch, 6158
6158
Each
$199.00
3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Rose Pink Tube, 27 inch, 6159
6159
Each
$199.00
3M™ Littmann® Cardiology IV™ Diagnostic Stethoscope, Standard-Finish Chestpiece, Burgundy Tube, 27 inch, 6184
6184
Each
$199.00
With any further questions you may have, please reach out to Bill Black.
Thank you,
Communication Type:
Product Update
Vendor #10296
-
06/3/2024
Title: Coloplast V#10581 - Biatain Ag Adhesive Product Discontinuatino
Details:
NAWSC_Biatain Ag Adhesive Discontinuation Letter_PM-31809.docx
Concordance Team,
The attached letter contains 5 wound care items Coloplast will be discontinuing effective June 1st. These items have been on a long-term backorder. According to the last backorder report they sent to Concordance, none of these items were on backorder to Concordance.
These items have been placed in item inactivation due to being discontinued in our system.
S#763052 Mfg#9632 disc by mfg-suggested sub s#223288 Mfg#39638 or S#728790 Mfg#9622
S#763250 Mfg#9635 disc by mfg- suggested sub Mfg#39640 (replacement not set up) or S#728808 Mfg#9625
S#158053 Mfg#9641 disc by mfg-suggested sub S#350573 Mfg#39651
With any further questions you may have, please reach out to Mary Reinhart.
Regards,
Communication Type:
Discontinuation Notice
Vendor #10581
-
05/31/2024
Title: Abbott V#10575 - Discontinuation Notice
Details:
SKU Rationalization Customer Letter. pdf
Abbott Discontintued Item Notice Cross Ref.
05-31-2024 Abbott Discontinued Item Usage
Concordance Team,
After careful consideration, Abbott has decided to discontinue certain products as outlined in the attached letter to help streamline the Abbott portfolio. This discontinuation timelines are based on inventory availability for each product. Some items have an immediate out of stock date as these items were deprioritized and unavailable in the market since 2023. Estimated out of stock dates for the remaining items vary and are based on current customer demand. Once inventories are exhausted, no additional quantities will be available.
Please see the attached letter and usage information for further details.
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Discontinuation Notice
Vendor #10575
-
05/30/2024
Title: Vitaflo V#12844 - Renastep Product Info
Details:
Renastep Quick Summary October 23
Concordance Team,
Our Vitaflo representative has provided us product information for Renastep, a ready to drink renal formula for ages >1yo. Refer to the attachment for further information.
With any further questions you may have, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Flyer
Vendor #12844
-
05/30/2024
Title: Abbott V# 10575 - Weekly Nutrition Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 05-28-2024. Also attached is a usage report for the Adult Institutional Items from the last 6 months. There was no usage information for the PSN Deprioritized items listed in the Inventory Letter.
-
- Please see the attachments listed below to reference this notification
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
-
05/29/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_24May2024
Nestle Weekly Inv. Update Item Cross Ref.
05-29-2024 Nestle Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 05-13-2024.
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
05/29/2024
Title: Safetec V#10044
Details:
Safetec Product Launch
Hi Bill,
Thank you once again for stopping by our table during last week’s NDC Exhibition down in Nashville. It was a pleasure meeting you. Hope you enjoyed the rest of your trip and had smooth travels home.
Per your request, I have attached a copy of our current linecard for your review. Dave is cc’d on this email as he manages the Concordance Account.
Please do not hesitate to contact me directly if there is anything I can personally do to help. We are excited to grow our partnership with you.
Best,
John Paul (JP) Sfeir
Director of Sales
716-895-1822 ext. 124
One Team, One Goal. Customer Driven.
Communication Type:
New Product Launch
Vendor # 10044
-
05/29/2024
Title: NDC V#10030 - Duracell (Procell) Product Update
Details:
PRO-343150-2022-Constant-SrAsst-Living_Sell_Sheet_v1
PRO-343501-2022-Constant-Healthcare_Sell_Sheet_v1
PRO-6090157949-2024_ProviderFlyer_v4
Concordance Team,
Advantages of Duracell / Procell:
Literature regarding the Intense Product line – This is the best battery for High Drain Devices. Also included is Segment specific items.
Plus – you may also find these helpful:
Infusion Pump https://youtu.be/zn-2bKbZDwc
Glucometer https://youtu.be/Uwo6jYMQv-Q
With any further questions you may have, please reach out to Bill Black.
Regards
Communication Type:
Product Update
Vendor #10030
-
05/29/2024
Title: MicroCare V#10201
Details:
MicroCare Steris Opportunity
Hi All,
Hope everyone had a great holiday weekend.
I am attaching an opportunity to convert Steris enzymatic and cleaners to Microcare. Microcare, formally Certol, signed a DA with us with favorable funding. I ran 6 month usage for the Steris items and broke them down on a pivot for easy insight.
Attached is also Microcare’s catalog and sales sheets for easy reference. All information will be posted in Hubspot.
Marianna, can you please load the subs in the system?
Contact myself or Charlie Campbell(information below) with any questions.
Charlie Campbell
Key Account Manager
Microcare LLC | 6120 East 58th Avenue Commerce City, CO 80022
Email: CharlieCampbell@MicroCare.com| Mobile: 720.812.2432
www.Microcare.com/certol | www.ACIDMagic.com
Thank you for all your efforts in the field! It is greatly appreciated!
Communication Type:
Product Catalog
Vendor 310201
-
05/24/2024
Title: Nestle V#10183 - Impact Peptide 1.5 Update
Details:
IMPACT Peptide 1.5 - Distributor Letter - Final.pdf
IMPACT Peptide 1.5 - Customer Letter - May 2024 - Final.pdf
05-24-2024 Nestle Impact Peptide 1.5 Item Usage
Concordance Team,
Nestle has made us aware of an inventory out of stock on Nestle's Impact Peptide 1.5. This is going to extend into 2025 and they have attached a customer letter with some suggested alternatives. Please see the attachments for further information.
With any further questions you may have, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
05/24/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 05-20-2024. Also attached is a usage report for the Adult Institutional Items from the last 6 months. There was no usage information for the PSN Deprioritized items listed in the Inventory Letter.
-
- Please see the attachments listed below to reference this notification
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
-
05/24/2024
Title: Jant V#10420 Product Catalog
Details:
I’ve attached the price list for 2024 as well as our point of care brochure and Vizient brochure.
Please let me if you have any questions regarding our product line or partner with Vizient.
Looking forward to seeing you in June!
Tiffany
Communication Type:
Product Catalog
V#10420
-
05/24/2024
Title: Coloplast V#10581 - Foley Catheter Supply Interruption
Details:
Folysil Customer Communication.pdf
Concordance Team,
Please see attached communication and below on product disruption from Coloplast effecting a portion of their Folysil Catheters. Attached is the official notice, customer usage and a list of all items for your use and reference.
With any further questions you may have, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10581
Communication Type:
Backorder Notification/Update/Reports
Vendor #10581
-
05/24/2024
Title: Detecto V#10793 - Sales Roster Update
Details:
WasteMate Comparison Chart.pdf
Concordance Team,
Detecto has provided a cross reference of thier new Waste Mate Models with the Rubbermaid SlimJim series. The new WasteMate models are set up in the NDC system, so sales reps can order immediately.
With any further questions you may have, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Update
Vendor #10793
Communication Type:
Product SKU List
Vendor #10793
-
05/23/2024
Title: Abbott V#10575 - Product Availability Update
Details:
05-23-2024 Abbott Product Availability Update Item Usage
Concordance Team,
Abbott Nutrition has provided an update that the following SKUs are available to order. These items will have quantities reviewed to ensure equitable access for patients across all channels. You may still see these items on the weekly inventory notifications. They are just communicating updates on availability.
MFG# VAI Item Item Description 63081 216400 Vital HP 1L RTH BTL 8CT 64820 248651 Vital HP 8oz CRTN ARC 24CT With any further questions you may have, please reach out to Mary Reinhart.
Thanks,
Communication Type:
Product Inventory Update
Vendor #10575
Communication Type:
Product Update
Vendor #10575
-
05/22/2024
Title: Cardinal V#10801 - Product Availability Update
Details:
ProductAvailabilityUpdateCAHBrand (1).pdf
Concordance Team,
Cardinal has provided a product availability update as of 05-17-2024. Please refer to the attachment above for further information.
Please reach out to Bill Black with any further questions you may have.
Regards,
Communication Type:
Product Inventory Update
Vendor #10801
Communication Type:
Product Update
Vendor #10801
-
05/22/2024
Title: Midmark V#10987 - Technical Service Communication
Details:
Concordance Team,
In order to streamline the process to promptly respond to a mutual customer with a service issue regardnig a Midmark product, see the below highlighted message. This will help with any future delays in responding to the customer.
“If you receive a call from a customer with a service issue regarding any Midmark products, please direct the customer to call 1-800-MIDMARK and press 1 for Midmark Technical Support. This is always the fastest path to resolution versus calling any other Midmark teammates. Thank you.”
With any further questions you may have, please reach out to Bill Black.
Regards,
Communication Type:
Updated Supplier Information
Vendor #10987
-
05/17/2024
Title: Medical Developments International V#13587 -
Details:
MDI VALUE ANNOUNCEMENT Compact Space Chambers 12-23
SC24001_SpringPosterA4_Draft3 - Consumer
Value Presentation MDI - Compact Space Chamber - PDF
Concordance Team,
Medical Developments International V#13587, a respiratory product supplier, has provided the further information on their products lines for accute and non-acute spaces. Please see the attachments above for further information.
With any further questions you may have, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Update
Vendor #13587
Communication Type:
Updated Supplier Information
Vendor #13587
-
05/17/2024
Title: Sekisui V#10161 - OSOM Promotions
Details:
Distributor Promotional Flyer (final).pdf
End User Promotional Fliyer (final).pdf
Concordance Team,
Sekisui is happy to announce their 2024 OSOM Rapid Respiratory Infection Test promotion for end users and rep spiffs, the Take - A - Breath campaign!
This promotional offering includes:
- OSOM Covid - 19 Antigen Rapid Test (cat. no. 1066-40)
- OSOM Ultra Plus Flu A&B Test (cat. no. 1032)
- OSOM Flu SARS - CoV - 2 Combo Test (cat. no.1080)
End User Promotion
This is fulfilled by Sekisui Diagnostics so there is nothing to do on your end except spread the word to your customers. Flyer is attached for dissemination to both your reps and end users.
Rep Spiff
To participate, we will need your approval and you will need to agree to the terms as outlined in the flyer.
With any further questions you have, please reach out to Bill Black.
Regards,
Communication Type:
End User Promo
Vendor #10161
-
05/17/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 05-13-2024. Also attached is a usage report for the Adult Institutional Items from the last 6 months. There was no usage information for the PSN Deprioritized items listed in the Inventory Letter.
-
- Please see the attachments listed below to reference this notification
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
-
05/17/2024
Title: Neslte V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_17May2024
Nestle Weekly Inv. Update Item Cross Ref.
05-13-2024 Nestle Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 05-13-2024.
- Nutren 2.0 1000 ml added
- Peptamen 1.5 1000 ml was added
- Vivonex 1000 ml was added
- Boost GC Vanilla was added
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
05/15/2024
Title: Hartmann V#10739
Details:
Attached are the Premier and HPG contracts, as well as the Premier Flyer that shows the products that are on contract. We were successful in getting some of our AWM products added to both contracts.
Please add these to your Hartmann contracts.
Thanks
Have an incredible day…………………..
Cliff Christianson
Sr. Director of Strategic Accounts
HARTMANN USA
Mobile: (909) 630-3678
Email: cliff.christianson@hartmann.info
Communication Type:
Product SKU List
Vendor #10739
-
05/15/2024
Title: Convatec V#10695 - Ostomy Conversion Opportunity
Details:
EB Key Acute Care SKUs & Hollister-Coloplast X-REF
EB Opportunity - Hollister & Coloplast Usage 6-mo
GPO OST Agreement List-- Esteem Body 20240412
Concordance Team,
Convatec has introduced their latest Ostomy Esteem Body product line - soft convexity ostomy barrier pouches. The products available to Concordance customers are now set-up in their system and present an opportunity to flip Hollister and Coloplast business. Please review the product materials below. A competitive cross-reference, GP details, and 6-month usage are attached for your review.
If you need to connect with your Convatec Sales Rep, attached is the latest roster.
Press Release: https://www.prnewswire.com/news-releases/convatec-launches-new-esteem-body-with-leak-defense-302117070.html?tc=eml_cleartime
Esteem Body eBrouchre: Esteem Body™ eBrochure .zip
Esteem Body video: Introducing Esteem Body™ with Leak Defense™ (2 mins).mp4
Esteem Body GPO Contracts: Attached.
Ostomy Digital Catalog: Ostomy Digital Product Guide.pdf
Suggested Acute Care Formulary for Esteem Body: Esteem Body™ Acute Formulary.pdf
Convatec Mfg Code
Concordance #
423651
394740
423652
394741
423656
394745
423664
394722
423665
394723
423666
394724
423689
394729
If you have any further questions, please reach out to Mary Reinhart.
Thanks,
Communication Type:
Sales Roster
Vendor #10695
Communication Type:
Product Update
Vendor #10695
Communication Type:
Product Inventory Update
Vendor #10695
-
05/15/2024
Title: Ansell V#11003 - MICRO-TOUCH Central Supply Glove Enhancements
Details:
Concordance Team,
Ansell is pleased to inform us of two enhancements to Ansell’s MICRO-TOUCH Central Supply SPD Plus ULNCS glove. First, the product shelf life has been updated from 3 to 5 years based on the results of our completed 5-year real-time shelf-life study. Second, it is now approved for the use with 15 chemotherapy drugs – up from 9. Please note that it is no longer recommended for use with Cytarabine. However, it is now also approved for protection against fentanyl. A chart detailing all drugs, breakthrough times, and differences can be found below.
Drug Name
Previous BTT (minutes)
NEW BTT (minutes)
Fentanyl
-
> 240
Carboplatin
-
> 240
Carmustine (BCNU)
1.3
24.0
Cisplatin
-
> 240
Cyclophosphamide (Cytoxan)
> 240
> 240
Cytarabine
> 240
-
Dacarbazine (DTIC)
-
> 240
Doxorubicin Hydrochloride
> 240
> 240
Etoposide (Toposar)
> 240
> 240
Fluorouracil
> 240
> 240
Ifosfamide
-
> 240
Methotrexate
> 240
> 240
Mitomycin C
-
> 240
Mitoxantrone
-
> 240
Paclitaxel
> 240
> 240
Thiotepa
67.8
56.9
Vincristine Sulfate
-
> 240
The new chemo information has been updated on the dispenser artwork, and the enhanced product featuring both the chemo and shelf-life improvements will appear in inventory starting warehouses in July.
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Update
Vendor #11003
-
05/14/2024
Title: Cardinal V#10801 - Holiday Closure (Memorial Day)
Details:
Holiday Closure Flyer - May 2024
Concordance Team,
Due to the upcoming holiday, Cardinal Health replenishment center will be closed for business on Monday, May 27th, 2024.
- Please place your orders on or before Wednesday, May 22nd before your normal cut-off time for delivery on your normal scheduled delivery date.
- Orders placed after your normal cut-off time on Wednesday will be processed on Tuesday May 28th.
- For standing delivery appointments (SDAs), adjustments will be coordinated by your transportation replenishment center leader.
If you have any further questions, please reach out to Bill Black for further assistance.
Best Regards,
Communication Type:
Holiday Schedule
Vendor #10801
-
05/10/2024
Title: Kate Farms V#12409
Details:
Kate Farms Std 1-4 Strawberry (May-2024 Launch Concordance)
KF to Nestle RTH OOS Cross Ref. (Distributors April 2024)
05-09-2024 KF to Nestle RTH OOS Item Usage
Concordance Team,
Given the recent announcement for Nestle OOS, attached is a cross-reference to share with reps and customers looking to continue using a closed - system. Kate Farms has plenty of inventory to immediately ship to our DCs.
Kate Farms is excited to introduce a new flavor option to our offering of a high-calorie, plant-based, organic nutrition, specially formulated for ages 14 and up. Standard 1.4 Strawberry will be available on May 21, 2024.
Please see the attachments for further information.
With any further questions you may have, please reach out to Mary Reinhart.
Best Regards,
Communication Type:
Product Update
Vendor #12409
Communication Type:
Product Inventory Update
Vendor #12409
-
05/10/2024
Title: Convatec V#10698
Details:
Hope you all had a great week. Please see attached announcement from Convatec. They have some packaging changes coming up. There will be no changes in product codes. Below are the effected items. I will also post in HubSpot for future reference and needs.
Please let me know if you have any questions or need additional information.
Communication Type:
Product Changes
VENDOR#10695
-
05/10/2024
Title: Abbott V#10575 - Weekly Nutrition Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 05-06-2024. Also attached is a usage report for the Adult Institutional Items from the last 6 months. There was no usage information for the PSN Deprioritized items listed in the Inventory Letter.
-
- Please see the attachments listed below to reference this notification
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
-
05/10/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_10May2024
Weekly Inv. Update Item Cross Ref.
05-06-2024 Nestle Weekly Nutrition Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 04-29-2024.
- Novasource Renal Vanilla was removed
- Novasource Renal Café Mocha was removed
- Boost Soothe was added
- Peptamen 1.5 1000 ml was added
- Fibersource 1000 ml and 1500 ml was added
- Novasource Renal 1000 ml was added
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
05/9/2024
Title: Abbott V#10575 - Product Inv. Update
Details:
05-09-2024 Product Availability Update Item Usage
Concordance Team,
Abbott Nutrition has provided an update that the following SKUs are available to order. These items will have quantities reviewed to ensure equitable access for patients across all channels. You may still see these items on the weekly inventory notifications. They are just communicating updates on availability.
MFG# VAI Item Item Description 64628 248641 JEV 1.5 CAL 8OZ CRTN ARC 24CT INST 64286 232578 ENS ENLIVE VAAN 8OZ BTL 24CT INST With any further questions you may have, please reach out to Mary Reinhart.
Thanks,
Communication Type:
Product Inventory Update
Vendor #10575
-
05/7/2024
Title: BD V#10125 - Allocation/Temp. Unavailability Update
Details:
Surgical Clippers BD Allocation Letter.pdf
BD Temp Unav- Customer Listing
Concordance Team,
BD has provided information stating that the two items below have been removed from Temp Una. and allocation so customers can begin ordering through us again. Stock requests will need to be reactivated as well.
MFG# VAI Item Description 5513E 208466 BD Surgical Clipper 5514A 208467 BD Surgical Clipper Charging Adapter With any further questions you may have, please reach out to Bill Black.
Regards,Communication Type:
Allocation Notification/Update
Vendor #10125
-
05/3/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 04-29-2024. Also attached is a usage report for the Adult Institutional Items from the last 6 months. There was no usage information for the PSN Deprioritized items listed in the Inventory Letter.
-
- Please see the attachments listed below to reference this notification
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
-
05/3/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_3May2024
Nestle Weekly Inv. Update Item Cross Ref.
05-06-2024 Nestle Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 04-29-2024.
- Boost Kid Essential 1.5 w/fiber was added
- Nutrisource Fiber Packets were added
- Vivonex 250 ml tetras were added
- Nutren 1.5 1000 ml were added
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
05/2/2024
Title: Hemocue V#10452 - Sales Roster Udpate
Details:
Updated HemoCue Field Sales Roster Public 2-25-2024
Concordance Team,
Attached is the most recent sales roster Hemocue has shared with us. Please see the attached file for further information.
With any further questions you may have, please reach out to Bill Black.
Regards,
Communication Type:
Sales Roster
Vendor #10452
-
05/2/2024
Title: Abbott V#10575 - Product Availability Update
Details:
05-01-2024 Product Availability Update Item Usage
Concordance Team,
Abbott Nutrition has provided an update that the following SKUs are available to order. These items will have quantities reviewed to ensure equitable access for patients across all channels. You may still see these items on the weekly inventory notifications. They are just communicating updates on availability.
MFG# VAI Item Item Description 64286 232578 ENS ENLIVE VAN 8OZ BTL 24CT INST 64905 248663 ENS PLUS VAN 8OZ CRTN ARC 24CT INST With any further questions you may have, please reach out to Mary Reinhart.
Thanks,
Communication Type:
Product Update
Vendor #10575
Communication Type:
Product Inventory Update
Vendor #10575
-
04/29/2024
Title: Myco V#11783 - Slide Deck
Details:
Concordance Team,
Please see the attached literature from MYCO.
Data.Syringe.Reli.ConventionalLuerLock. Rev 1
Data.Needle.Reli.Blunt Fill.Rev5
Data.BC.Reli.Needle.Safety.Rev0
If you have any further questions, please reach out to Mary Reinhart.
Best Regards,
Communication Type:
Product Update
Vendor #11783
-
04/29/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_26Apr2024
Weekly Inv. Update Item Cross Ref.
04-29-2024 Nestle Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 04-22-2024.
- Boost Kids Essentials Strawberry was added
- Peptamen Jr 1.5 was added
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10575
-
04/29/2024
Title: Hollister V#10901 - Continence Care Update
Details:
CC_US_VaPro_DistributionLetter_Email_0424
Concordance Team,
Please see the attached communication regarding a change to the Global Trade Item Number (GTIN) on boxes of Hollister's VaPro Pocket No Touch Intermittent Catheter. The attached distribution letter provides relevant details on this change and its timing.
With any further questions you may have, please reach out to Bill Black.
Best Regards,
Communication Type:
Product Update
Vendor #10901
Communication Type:
Product SKU List
Vendor #10901
-
04/26/2024
Title: MYCO V#11783 - Suture Guide
Details:
MYCO Suture guide
Happy Friday! Hope you all had a fantastic week.
MYCO Medical has shared their full line of sutures with a cross reference guide. Part of the offering by MCYO are a few solutions to the J&J discontinued sutures. They also have a new rep, Tom Carter, who is available to help with any questions or needs. His contact info is below and in the attached.
I have attached the MYCO cross reference file along with usage along with the corresponding discontinued J&J codes which are highlighted. Also attached are a suture catalog and data sheet for your reference and needs. All this information will be posted in HUBSPOT for you reference in the future. Marianna has also loaded all subs into VAI.
Please let me know if you have any additional questions or needs. I am more than happy to assist.
Tom Carter, tcarter@mycomedical.com or his mobile # 574-527-7745.
Thank you for your continued support and efforts in the field, it is truly appreciated.
Communication Type:
Product Catalog
Vendor #11783
-
04/26/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Concordance Team,
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 04-22-2024. Also attached is a usage report for the Adult Institutional Items from the last 6 months. There was no usage information for the PSN Deprioritized items listed in the Inventory Letter.
-
- Please see the attachments listed below to reference this notification
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
-
04/26/2024
Title: Abbott V#10575 -
Details:
Nestle Abbott RTH Cross Reference PDF
Nestle - Abbott RTH Product Conversion Cross Ref.
04-26-2024 RTH Product Conversion- Nestle Item Usage
Concordance Team,
Abbott has provided suggested Abbott RTH alternatives to Nestle UltraPak items that are temporarily out-of-stock as communicated by Nestle earlier this month. In the event we run low on inventory and need alternatives to fill customer orders, please refer to the cross reference file attached and shown below with our CHS#'s included.
Nestle Product Codes Abbott Item Codes MFG# VAI Item MFG# VAI Item MFG# VAI Item 14180100 198455 62685 197952 62683 197954 4390061715 385699 62713 197983 4390034927 356615 62683 197954 62681 197956 4390036583 197808 62675 197950 62677 197947 36508100 155392 62677 197947 62679 197951 4390018583 192852 62667 197955 62683 197954 18580100 154677 62683 197954 4390097371 183417 62719 197971 4390028182 196041 62689 197957 62681 197956 18180100 154676 62681 197956 62689 197957 18480100 154669 62697 197962 35180100 156019 62669 197958 9871677400 149521 67405 296718 9871626354 144684 62699 197964 62697 197962 9871644146 141418 68048 334516 9871676390 142379 62715 197984 9871618543 144877 67419 296597 9871677360 149522 67415 296608 4390034957 195585 62713 197983 9871628194 142031 62713 197983 4390049322 234576 62711 197981 9871622804 137338 62711 197981 62715 197984 36280100 156625 No Abbott Alternative If you have any further questions, please reach out to Mary Reinhart.
Best Regards,
Communication Type:
Product SKU List
Vendor #10575
-
04/25/2024
Title: Solventum (PKA 3M Healthcare) V#10296 - N95 Status Update
Details:
Concordance Team,
Please see the latest update from Solventum (PKA 3M Healthcare) on N95 mask lead times. Allocations are still in place in our system until further notice.
Adding a few clarifiers since we've seen many of our DC's Carrying inventory to cover the long lead times.
- All allocations are removed
- Please work with customers for any increased demand due to fit testing or stock building and submit stock requests as appropriate
- This will be the last N95 supply update at this time
/04-25-2024Solventum%20(PKA%203M)%20N95%20Lead%20Time%20Update.png?width=604&height=255&name=04-25-2024Solventum%20(PKA%203M)%20N95%20Lead%20Time%20Update.png)
With any further questions you my have, please reach out to Jamie Yolles.Best Regards
Communication Type:
Product Inventory Update
Vendor #10296
-
04/25/2024
Title: Hollister V#10901 - Continence Care Update
Details:
CC_US_Onli Distributor Letter_Catheter Effective Length Change_0424
04-26-2024 Continence Care Updates Item Usage
Concordance Team,
Please see the attached communication from Hollister regarding the effective length of Onli™ Intermittent Catheter.
The effective length of the catheter has been reduced by 1cm/10mm/0.4". The term "catheter effective length" refers to the length of the catheter that can be inserted into the urethra. The change does not affect the performance or the intended use of the product. In addition, nothing is changing relative to the box/case dimensions, or weights.
This change will go into effect in April 2024 and consumer change notifications will be included in boxes starting in April 2024.

If you have any further questions, please reach out to Bill Black.
Best Regards,
Communication Type:
Product Changes
Vendor #10901
-
04/24/2024
Title: Abbott V#10575 - Product Availability Update
Details:
04-24-2024 Product Availability Update Item Usage
Abbott Nutrition has provided an update that the following SKUs are available to order. These items will have quantities reviewed to ensure equitable access for patients across all channels. You may still see these items on the weekly inventory notifications. They are just communicating updates on availability.
Attached above is a usage report for the last 6 months including the SKU's listed below. Please note CHS#176925 has had no sales since early 2023.
MFG# CHS# Item Description 58301 176925 ENS PLUS STR 8OZ BTL 24CT INST 62667 197955 JEV 1.2 CAL 1.5L RTH BTL 6CT With any further questions you may have, please reach out to Mary Reinhart.
Thanks,
Communication Type:
Product Inventory Update
Vendor #10575
Communication Type:
Product Update
Vendor #10575
-
04/24/2024
Title: ABS V#12222 - Contact Sheet Update
Details:
Concordance Team,
We have an updated sales roster from Horizon Scientific, the parent company of Corepoint Scientific, Standex Scientific Solutions and American Biotech Supply. Please see the attached contact sheet/sales roster for further information.
If you have any further questions, please reach out to Jeff Shook.
Best Regards,
Communication Type:
Sales Roster
Vendor #12222
-
04/19/2024
Title: Stryker V#11036 - SSS New ERP system and Procecss
Details:
Concordance Team,
Stryker Sustainability Solutions has notified us they will be launching their new enterprise resource planning (ERP) system and process on May 6, 2024. When this happens you will notice changes to their sales process and the overall look of their documents.
Please view the attached communication to learn more about the changes you can expect when the new ERP launches.
With any further questions you may have, please reach out to Jamie Yolles.
Best Regards
Communication Type:
Updated Supplier Information
Vendor #11036
-
04/19/2024
Title: Abbott V#10575 - Weekly Inv. Update
Details:
Abbott Nutrition has shared with us the attached Abbott Nutrition inventory updates for the week of 04-15-2024. Also attached is a usage report for the Adult Institutional Items from the last 6 months.
-
- Please see the attachments listed below to reference this notification
If you have any further questions, please reach out to Mary Reinhart.
Thank you!
Communication Type:
Product Inventory Update
Vendor #10575
-
-
04/19/2024
Title: Nestle V#10183 - Weekly Inv. Update
Details:
NHSc Weekly Inventory Letter_19Apr2024
Weekly Inv. Update Item Cross Ref.
04-22-2024 Nestle Weekly Inv. Update Item Usage
Concordance Team,
Attached are the Nestle Inv. Updates from the week of 04-15-2024.
- Isosource 1.5 1000 ml and 1500 ml added
- Diabetisource 1000 ml and 1500 ml added
- Fibersource 1000 ml and 1500 ml added
- Peptamen AF 1000 ml added
- Vivonex 250 ml added
- Boost Kids Essential Vanilla removed
- Boost Kids Essential Chocolate Craze removed
- Boost Very High Calorie Vanilla removed
If you have any further questions, please reach out to Mary Reinhart.
Regards,
Communication Type:
Product Inventory Update
Vendor #10183
-
04/18/2024
Title: Abbott V#10575 - Product Availability Updated
Details:
04-18-2024 Abbott Product Availability Update Item Usage
Abbott Nutrition has provided an update that the following SKUs are available to order. These items will have quantities reviewed to ensure equitable access for patients across all channels. You may still see these items on the weekly inventory notifications. They are just communicating updates on availability.
MFG# CHS# Item Description 58299 176941 ENS PLUS CHO 8OZ BTL 24CT INST 62719 197971 PIVOT 1.5 CAL 1L RTH BTL 8CT With any further questions you may have, please reach out to Mary Reinhart.
Thanks,
Communication Type:
Product Inventory Update
Vendor #10575
Communication Type:
Product Update
Vendor #10575
-
04/18/2024
Title: BDV#10125
Details:
Q2 PROMO BD
Good morning Bill – just returned from PTO and wanted to get these Q2 promotions to you.
NATIONAL PROGRAMS:
- Heart Health Resting ECG & Vitals Offer- Excited to bring you a new type of offer with this program!
- The customer receives a spirometry module at no charge when they purchase a CP 150 or a Diagnostic Cardiology Suite Resting ECG!
- To receive spirometry module- Customer first needs to purchase the resting ECG only, then go to the redemption link to fill out the form and attach an invoice. Spiro module will be sent directly to the customer.
- This promotion also includes a $150 rebate on the Spot 4400 & CSM!
- Please note- there are 2 separate redemption pages for the vitals and spirometry offers.
- Exam Tools Trade up Offer- Another brand-new offer we are happy to launch! We will provide an end-user rebate to any customer who wants to "trade up" their legacy Welch Allyn devices to our newer “BEST” configurations:
- Rebates on “BEST” configurations only.
- Customers DO NOT need to send in old devices but will need to provide a picture of what "they're trading in" on the rebate form.
- This is open to all customers, excluding medical schools.
- No max on rebates per customer!
- Modernize Your Q-Stress Equipment Offer- Large rebates on Q-Stress!
- $2,000 rebate with a purchase of the Q-Stress Testing System & Tango Automated BP Device from SunTech!
- $1,000 rebate with a purchase of the Q-Stress Testing System
- Spot Vision Rebate Offer- We increased our offer for Q2 to $750!
Attached are the fliers for these promotions. I know that our teams will be actively engaged as we work these new programs for Q2. All the best
Communication Type:
End User Promo
V#10125
- Heart Health Resting ECG & Vitals Offer- Excited to bring you a new type of offer with this program!
-
04/16/2024
Title: Convatec V#10695 - Sales Roster Update
Details:
Convatec Field Sales Roster 04-01-2024
Sales Force Lookup by Zip Code
Concordance Team,
Convatec has provided us with their most up to date sales roster from 04-01-2024. Please see the attachments above for further information.
With any further questions you may have, please reach out to Valeriya Stoyanova.
Best Regards,
Communication Type:
Sales Roster
Vendor #10695
-
04/16/2024
Title: PDI V#10497 - Vizient Contract Announcement
Details:
Profend Vizient Info Summary Sheet_03249011
PDI Profend Nasal Decolonization Brochure_01217247
Profend Patient Education Pamphlet_08190785
Concordance Team,
PDI is please to announce as of 04-01-2024, Profend Nasal Decolonization has been added to Vizient agreement #MS1316. Please see the attachments above for further information.
For any further questions you may have, please reach out to Jamie Yolles.
Thank you,
Communication Type:
Updated Supplier Information
Vendor #10497
-
04/15/2024
Title: Cardinal Health - Product Availability and Lead Times
Details:
Concordance team,
Cardinal has provided their latest update on all of their categories. Please see the attached and feel free to share the status and recovery dates with customers, as you may be working on constrained products/categories.
Enteral Feeding
Presource® Procedural Packs
Facial Protection
Sharps Safety
Fluid Management
Skin Management
Exam Gloves
Surgical Drapes & Gowns
Incontinence
Surgical Essentials
Infection Control Apparel
Surgical Gloves
Laboratory Products
Traditional & Advanced Wound Care
Localized Temperature Therapy
Urology
Medical Essentials
Women & Baby
Medicated Wipes
Wound Drains
OR Accessories
For more supplier news and updates, visit the Supplier Communication Hubspot page.
Have a great day,
Supplier Relations
Communication Type:
Product Inventory Update
Vendor #10801
-
04/15/2024
Title: Molnlycke V#10008 - New Product Launch- Biogel® PI Micro Indicator® System
Details:
Concordance Team,
Mölnlycke Health Care, manufacturer of Biogel surgical gloves, is please to introduce a new synthetic double-gloving system, the Biogel PI Micro Indicator System that offers safety, comfort, efficiency, and sustainability – all in one convenient package.
The Biogel® PI Micro Indicator® System offers:
Improved convenience
- A single pack containing two pairs of gloves designed and sized specially to glide on easily and work perfectly together.
Improved sustainability
- Convenient double-packed gloves with 50% less plastic packaging by eliminating a sterile pouch and packing in smaller boxes.
Improved protection
- Consists of a blue, polyisoprene surgical indicator underglove and a straw-colored overglove, creating a patented puncture indication system proven to provide clear, fast and large1 visual alert to the wearer reducing the risk of occupational exposure.
Improved comfort
- Biogel® PI Micro Indicator® System gloves are extra thin and conform to the hand for maximum tactile sensitivity when double gloving.
- ½ size larger underglove enhances comfort.
Biogel® PI Micro Indicator® System (48355–48385) features:
- Sterile, synthetic, polyisoprene surgical glove (manufactured without natural rubber latex).
- Contains blue underglove ½ size larger than overglove, tested for use with chemotherapy agents.
- Contains straw color overglove, tested for use with chemotherapy agents.
- Made with a curved former for a more natural fit.
- Formulated for improved sensitivity even when double gloving.
- Standard grip and anti-slip overglove cuff.
Recommended use
- Recommended for all surgical procedures and especially those when extra tactile sensitivity is desired even when double gloving.
Attached is the Biogel® PI Micro Indicator® System launch letter, as well as the Biogel® PI Micro Indicator® System data sheet for your review. Please contact your local representative or call the Molnlycke Customer Service Center at 1-800-843-8497 for more information.
Communication Type:
New Product Launch
Vendor #10008
-
04/12/2024
Title: Nestle V#10183 - Formula Packaging Communication
Details:
UltraPak Customer Letter2 NEW 4.12.24
UltraPak to Tetra carton Cross Reference 4.12.2024
04-19-2024 Nestle UltraPak Item Usage
Concordance Team,
Please see attached important update regarding Nestle's formula packaging for UltraPak.
In an ongoing commitment to supporting the needs of more patients, Nestlé is implementing measures to strengthen their UltraPak production capabilities. This is their formula that is in 1000mL and 1500mL packaging. While improvements are being made, they will experience temporary out of stocks in the 1000mL and 1500mL packaging. Specific dates and details are included in the attached letter (1st attachment).
Fortunately, each of their formulas in the 1000mL and 1500mL packaging has the exact same formula that will remain available in their Tetra Prisma cartons. Nestle has committed a smooth transition and providing any support needed during this time. The 2nd attachment is a cross-reference that includes our UltraPak items (on the left) that will experience temporary out of stocks and the exact same product in the Tetra Prisma cartons (on the right) that will remain available and can be the replacement during this time.
Please reach out to Mary Reinhart with any further questions you may have.
Thank you!
Communication Type:
Product Update
Vendor #10183
-
04/11/2024
Title: J&J Sutures Discontinuation
Details:
Concordance team,
Many of you have been aware of the J&J Suture discontinuation that was to be effective this April. Bill has actively been working with the supplier regarding some of your concerns this may cause with customers. Please know that J&J will continue to fill orders until their stock is depleted on the old items. The substitutes listed in the attached file are just that – customers need to review and approve each one before we can bring in additional products. Attached is a 2024 YTD sales history of these affected items.
See the message from Bill below:
All,
Discussion with my J&J contact on this discontinuation, you will need to have your customer reach out to their local J&J representative to work through this change and make sure suggested substitutions are approved. If you know your local rep please make contact with them to move this along.
Please let me know if you have any roadblocks with J&J representatives.
Internally the team is working on the system and updating as current items are no longer available and suggested subs are entered.
Let me know if you have any questions, very important that you and the J&J local rep are engaged with the customer.
Thank you!!
--------------------------------------------------
Bill Black | (Supplier Relations) Director, Channel Optimization
Concordance Healthcare Solutions
85 Shaffer Park Drive, Tiffin, OH 44883
419-455-2022 | bblack@concordancehs.comCommunication Type:
Discontinuation Notice
Vendor #10196
-
04/11/2024
Title: Cardinal V# 10801 - Product Availability Update
Details:
Product Availability Update CAHBrand 3.29.2024
Concordance Team,
Please see the attached product availability update from Cardinal.
If you have any further questions, please reach out to Bill Black.
Regards,Communication Type:
Product Inventory Update
Vendor #10801
Communication Type:
Product Update
Vendor #10801
-
04/10/2024
Title: Essendant V#10572 - Recall Notice
Details:
Concordance Team,
Please see the important recall notice from Essendant below!
IMPORTANT MESSAGE REQUIRING YOUR IMMEDIATE ATTENTION
Product Recall Notice on Select Lot Codes of Tide PODS® Laundry Detergent
Dear Valued Customer,
Essendant is alerting you to a product recall announced by Procter & Gamble affecting certain lot codes of Tide PODS Laundry Detergent sold by Essendant under the item numbers: PGC93126CT, PGC93126EA, PGC93127CT, and PGC93120. To view the list of impacted lot codes sold by Essendant, please click here.
Additional information about this recall can be found in the manufacturer announcement, which you can find here.
Please refer to this announcement for important information regarding the recall, including actions you may need to take with regard to affected product.
This product recall only impacts products identified in the manufacturer announcement.
Customer contact for questions about this recall and how to request a refund for impacted products: Call Procter & Gamble toll-free at 833-347-5764 from Monday through Friday, 9 AM ET to 6 PM ET, Saturday, 9 AM ET to 5:30 PM ET, or online at www.pg.com/bags.
Thank you for your prompt compliance.
Sincerely,
The Essendant Team
With any further questions you may have, please reach out to Jeff Shook.
Thank you,
Communication Type:
Recall Notification
Vendor #10572
-
04/10/2024
Title: 3M V#10296 - Name Change to Solventum
Details:
EIN W9 Tax Customer Update Feb. 2024 pdf
Solventum Channel Communication_April 2024-Final
Concordance Team,
3M Healthcare has changed its legal entity structure, resulting in a change to the federal employer ID Number and the associated W-9 form of the selling entity that we purchase from, 3M Healthcare US OpCo LLC. Please see the attachments for further information.
Old Name: 3M Healthcare US OpCo LLC V#10296
New Name: Solventum Corporation V#10296
The system has been updated accordingly, please update your systems as needed.
Thank you,
Communication Type:
Updated Supplier Information
Vendor #10296







